To determine whether rheumatoid arthritis (RA) patients who have been prescribed biological agents exhibit a different comorbidity burden than RA patients who take disease-modifying antirheumatic drugs (DMARDs) alone, and to understand the association between comorbidity and other variables, as well as the association between comorbidity and multimorbidity.
MethodsThis observational case–control study included 114 RA patients treated with biological agents and a control group comprising 163 sex- and age-matched RA patients treated with DMARDs only. Current and previous data regarding the patients’ disease activity, comorbidities, and treatments were collected. The data were analysed using bivariate and multivariate regression models.
ResultsThe patients who were prescribed biological agents exhibited poorer disease control, received more DMARDs and steroids, and underwent more total joint arthroplasties compared with the patients in the control group. However, the risk factors for cardiovascular disease and the comorbidity frequency were similar between cases and controls. The most prevalent comorbidities were hypertension, obesity, and respiratory, thyroid, and upper gastrointestinal disorders. The incidence of cardiovascular disease was low, and only 29% of the patients exhibited multimorbidities. A bivariate association of age, late diagnosis, joint replacements and a high score on the health assessment questionnaire score (HAQ) with comorbidity was observed. There were also correlations between the Charlson index and age, joint reconstructive surgery, disease activity (DAS28), and HAQ score. However, when binary logarithmic regression models were applied, only patient age remained significantly associated with comorbidity and multimorbidity [hazard ratio, 1.08; 95% confidence interval, 1.05–1.12; p<0.0005].
ConclusionRA patients taking biological drugs have a comorbidity burden equivalent to those treated with DMARDs alone. Age is the main predictive factor of comorbidity in these patients.
Determinar si los pacientes con artritis reumatoide (AR) a los que se les prescribe terapia biológica tienen comorbilidad diferente a los pacientes con AR a los que se les prescribe solo fármacos antirreumáticos modificadores de la enfermedad (FAME). Entender la asociación de comorbilidad con otras variables y con multimorbilidad.
MétodosEstudio observacional de casos y controles, incluyó 114 pacientes con AR a los que se les prescribió terapia biológica, y un grupo control de 163 pacientes emparejados por sexo y edad a los que solo se les había prescrito FAME. Se recogieron datos previos y actuales sobre actividad de enfermedad, comorbilidad y tratamientos. Se realizó análisis de regresión bivariante y multivariante.
ResultadosLos pacientes a los que se les prescribió terapia biológica tenían: peor control de la enfermedad, recibieron más FAME y glucocorticoides y se habían sometido a más artroplastias en comparación con el grupo control. Sin embargo, los factores de riesgo cardiovascular y la frecuencia de comorbilidad fueron similares entre casos y controles. Las comorbilidades más frecuentes fueron: hipercolesterolemia (33%), hipertensión (27%), obesidad (26%), y trastornos respiratorios (16%), tiroideos(13%) y gastrointestinales (10%). La incidencia de enfermedad cardiovascular es baja (2%). Solo el 29% de los pacientes tenían multimorbilidad. Se observó asociación bivariante entre edad, diagnóstico tardío, reemplazos articulares y HAQ, con comorbilidad. También se observaron correlaciones entre índice de Charlson y edad, la cirugía reconstructiva, actividad de la enfermedad y HAQ. Cuando se aplican los modelos de regresión Log binario, solo la edad se mantuvo asociada significativamente con comorbilidad y multimorbilidad (hazard ratio 1,8; intervalo de confianza al 95% 1,05-1,12; p<0,0005).
ConclusiónLos pacientes con AR con terapia biológica tienen comorbilidad equivalente a los tratados solo con FAME. La edad es el principal factor predictivo de comorbilidad en estos pacientes.
Rheumatoid arthritis (RA) is a relatively common autoimmune disease that causes progressive health impairment. Its prevalence in the general population of Spain is 0.5%,1 whereas the number of new cases arising annually is approximately 8.3 cases per 100,000 people (95% confidence interval (CI): 7.5–9.2).2 RA not only impairs the musculoskeletal system but can also affect the entire body and increase morbidity and mortality. Both age and physical disability affect health-related quality of life and the costs associated with managing the disease.3 The coincidence of two or more diseases in a patient with RA, a phenomenon known as multimorbidity, worsens the health-related quality of life as well as the major health outcomes of RA, including the costs of coexisting disease.4 In fact, the rate of disability increases with the number of comorbid diseases.5 Moreover, the number of comorbidities is an independent risk factor for premature death6 and drug toxicity7 in RA patients.
Because early and aggressive control of the inflammation associated with RA results in improved survival,8,9 the addition of new biological agents in the treatment of RA could help to reduce mortality. However, fewer than 30% of patients with RA currently take biological agents.7 The patients who receive anti-TNF therapies often have the most severe disease, with high levels of disability. However, patients with high levels of comorbidity are rarely prescribed anti-TNF therapy but may remain on standard DMARD therapies alone.There are few studies that analyse comorbidities in patients with RA who will receive biological therapy. Furthermore, there are no specific studies employing the design used in our study to compare comorbidities in patients with or without biological drugs. As RA is a prevalent disease, it is important to consider comorbidities; in our opinion, it is critical to impart these findings to the medical society.
Our aim was to determine whether RA patients who have been prescribed biological agents to control their inflammatory activity have a comorbidity burden different from those who were prescribed disease-modifying antirheumatic drugs (DMARDs) and to understand the associations between comorbidity and other variables and between comorbidity and multimorbidity.
MethodsThis observational case–control study was performed in a tertiary care centre (reference population of 628,912 inhabitants; current annual load of approximately 1500 follow-up RA patients). All the patients who entered the cohort signed a written consent form after being informed regarding the details of the study, according to the Declaration of Helsinki and Spanish research regulations. The study protocol was also reviewed and approved by the centre's ethical committee.
CasesThe study sample comprised 114 patients with RA involved in a longitudinal study initiated in March 1999.10 All the consecutive patients with RA (according to the ACR criteria)11 who failed to at least two DMARDs, including methotrexate, and who, in the opinion of their respective doctors, required or were prescribed biological therapy between May 2006 and December 2008 were included in the study. Only biologically naïve patients were included.
ControlsWe included a control group that comprised 163 sex- and age-matched RA patients prescribed DMARDs who had never received biological treatment. The controls were recruited from the same outpatient clinic in a cross-section in 2009.
Study protocolThe participating rheumatologists collected data from the patients during structured visits. The patients and controls provided informed consent prior to receiving treatment. All the cases were systematically evaluated for tuberculosis following the recommendations of the European and Spanish drug agencies.12 The following factors were analysed: sociodemographic variables, such as age, gender, and current smoking habits; data related to the disease, including the date of diagnosis, duration, rheumatoid factor, orthopaedic surgery, etc.; C-reactive protein (CRP) levels; erythrocyte sedimentation rate (ESR); current and previous treatments with DMARDs and corticoids; and comorbidities. The clinical diagnoses were assessed according to self-reported history and reviews of clinical records, including current and previous treatments.
All the participants were clinically assessed upon their entry into the study, and data concerning clinical activity and physical function were collected, as were blood and urine specimens. The disease activity was estimated by the 28-joint disease activity calculator (DAS28-ESR)14,15 and DAS28-CRP.16 Physical function was evaluated by a Spanish version of the Health Assessment Questionnaire (HAQ).17
Operational definitions of comorbidityThe comorbidities listed in the Charlson index13 and traditional cardiovascular risk factors (smoking, obesity, hypertension, diabetes, hypercholesterolaemia, family history of premature cardiovascular disease, and inactive lifestyle) were collected. Other comorbidities were collected including upper gastrointestinal bleeding or perforation, fibromyalgia, thyroid diseases, and respiratory disorders that restrict mobility.
Obesity was defined as a body mass index greater than 30kg/m2. Dyslipidaemia was defined by ATP III18 as a total cholesterol greater than 240mg/dl, LDL-cholesterol greater than 160mg/dl, HDL-cholesterol lower than 40mg/dl, and a triglyceride level greater than 200mg/dl. Additionally, patients requiring lipid-lowering drugs were also considered to have dyslipidaemia. Diabetes mellitus was defined according to the American and European Association for Diabetes19 as a fasting glucose level greater than 126mg/dl or a blood glucose level above 200mg/dl at any time of day. An active lifestyle was defined as being engaged in regular, low- to moderate-intensity physical activity for 20–30min/day, 3 days/week.20 The comorbidities were defined according to definitions outlined in the Charlson index.21 Multimorbidity was defined as the concurrence of two or more chronic conditions in addition to RA. Health conditions were considered to be chronic if they had been present for at least 3 months.
Statistical analysisThe quantitative descriptive data are presented as mean±standard deviation (SD). The qualitative variables are presented as percentages (%). Comparisons of the quantitative variables between cases (the RA patients who were prescribed biological agents) and controls (the RA patients who were prescribed DMARDs), were performed with Student's t-tests for independent samples or Mann–Whitney U-tests, when appropriate. Normality was confirmed by Kolmogorov tests. The Chi-squared or Fisher's exact two-tailed test was used to compare categories between the cases and the controls. We determined the univariate relationships of the variables of disease at the baseline visit with the Charlson index using Spearman's correlation. A two-tailed test was used, and a p<0.05 was considered to be significant. All the variables with p<0.10 in the bivariate analysis were entered into a logistic regression model (step forward/Wald test p=0.05, output p=0.10) in which the dichotomous dependent variables chosen included comorbidity and multimorbidity.
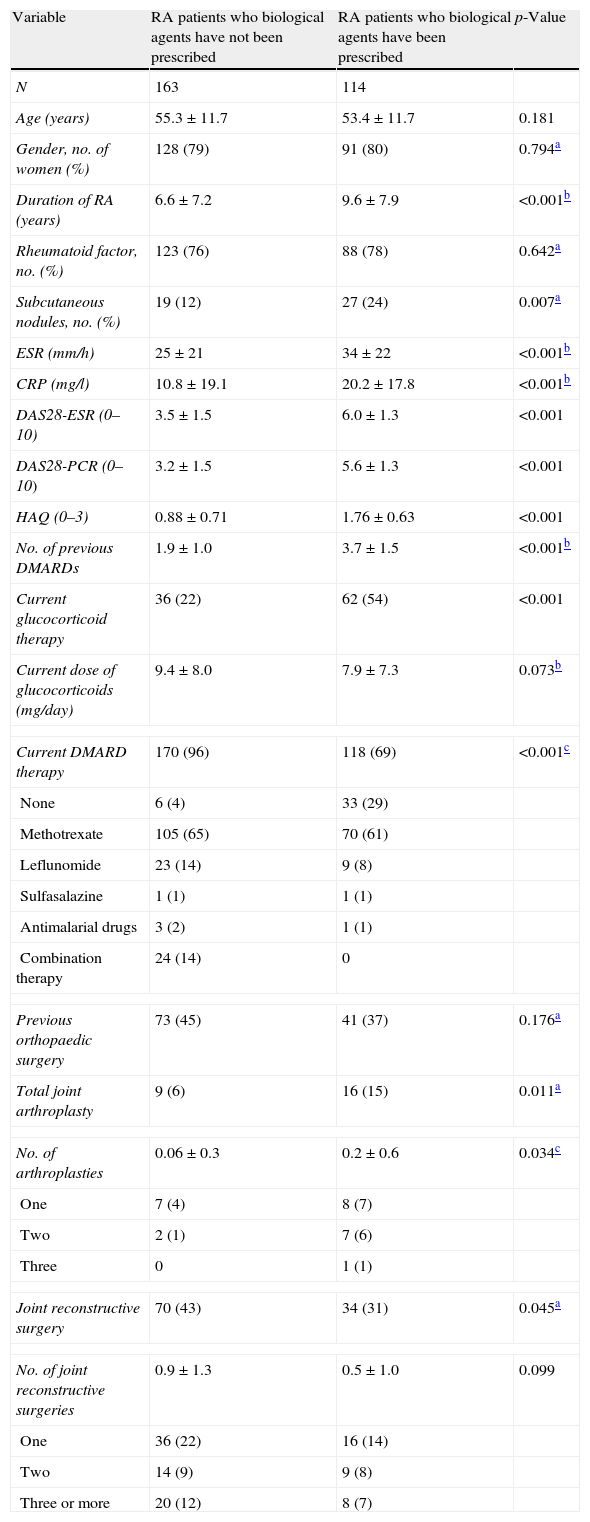
ResultsThe clinical status of the cases and controls is shown in Table 1. The mean age (SD) of the RA patients (79% women) was 54.6 (11.8) years (range, 16–80; median 56), and the mean disease duration was 7.9 (7.7) years.
Demographic and disease characteristics in RA patients who were prescribed biological therapy compared with patients who had not received biological therapy.
| Variable | RA patients who biological agents have not been prescribed | RA patients who biological agents have been prescribed | p-Value |
| N | 163 | 114 | |
| Age (years) | 55.3±11.7 | 53.4±11.7 | 0.181 |
| Gender, no. of women (%) | 128 (79) | 91 (80) | 0.794a |
| Duration of RA (years) | 6.6±7.2 | 9.6±7.9 | <0.001b |
| Rheumatoid factor, no. (%) | 123 (76) | 88 (78) | 0.642a |
| Subcutaneous nodules, no. (%) | 19 (12) | 27 (24) | 0.007a |
| ESR (mm/h) | 25±21 | 34±22 | <0.001b |
| CRP (mg/l) | 10.8±19.1 | 20.2±17.8 | <0.001b |
| DAS28-ESR (0–10) | 3.5±1.5 | 6.0±1.3 | <0.001 |
| DAS28-PCR (0–10) | 3.2±1.5 | 5.6±1.3 | <0.001 |
| HAQ (0–3) | 0.88±0.71 | 1.76±0.63 | <0.001 |
| No. of previous DMARDs | 1.9±1.0 | 3.7±1.5 | <0.001b |
| Current glucocorticoid therapy | 36 (22) | 62 (54) | <0.001 |
| Current dose of glucocorticoids (mg/day) | 9.4±8.0 | 7.9±7.3 | 0.073b |
| Current DMARD therapy | 170 (96) | 118 (69) | <0.001c |
| None | 6 (4) | 33 (29) | |
| Methotrexate | 105 (65) | 70 (61) | |
| Leflunomide | 23 (14) | 9 (8) | |
| Sulfasalazine | 1 (1) | 1 (1) | |
| Antimalarial drugs | 3 (2) | 1 (1) | |
| Combination therapy | 24 (14) | 0 | |
| Previous orthopaedic surgery | 73 (45) | 41 (37) | 0.176a |
| Total joint arthroplasty | 9 (6) | 16 (15) | 0.011a |
| No. of arthroplasties | 0.06±0.3 | 0.2±0.6 | 0.034c |
| One | 7 (4) | 8 (7) | |
| Two | 2 (1) | 7 (6) | |
| Three | 0 | 1 (1) | |
| Joint reconstructive surgery | 70 (43) | 34 (31) | 0.045a |
| No. of joint reconstructive surgeries | 0.9±1.3 | 0.5±1.0 | 0.099 |
| One | 36 (22) | 16 (14) | |
| Two | 14 (9) | 9 (8) | |
| Three or more | 20 (12) | 8 (7) | |
RA, rheumatoid arthritis; DAS28-ESR, 28-joint disease activity – erythrocyte sedimentation rate; DAS28-CRP, 28-joint disease activity – C reactive protein; HAQ, Spanish version of the Health Assessment Questionnaire; DMARDs, disease-modifying antirheumatic drugs.
The biological drugs available in our centre were: infliximab, etanercept, adalimumab, and rituximab.
The values for continuous variables are reported as the means±SD.
Compared with patients who were prescribed only DMARDs, the cases had the disease for a longer time and exhibited a higher frequency of subcutaneous nodules, an increased inflammatory activity, and worse physical function. These patients had also previously received a greater number of DMARDs, and a higher proportion was not currently using DMARDs. Furthermore, more than half of the study groups were receiving corticosteroids. Total joint arthroplasties were twice more common in the RA patients requiring biological therapy than in the patients taking only DMARDs. Only certain patients who were not prescribed biological agents received combination DMARD therapy. Other characteristics did not differ between the groups.
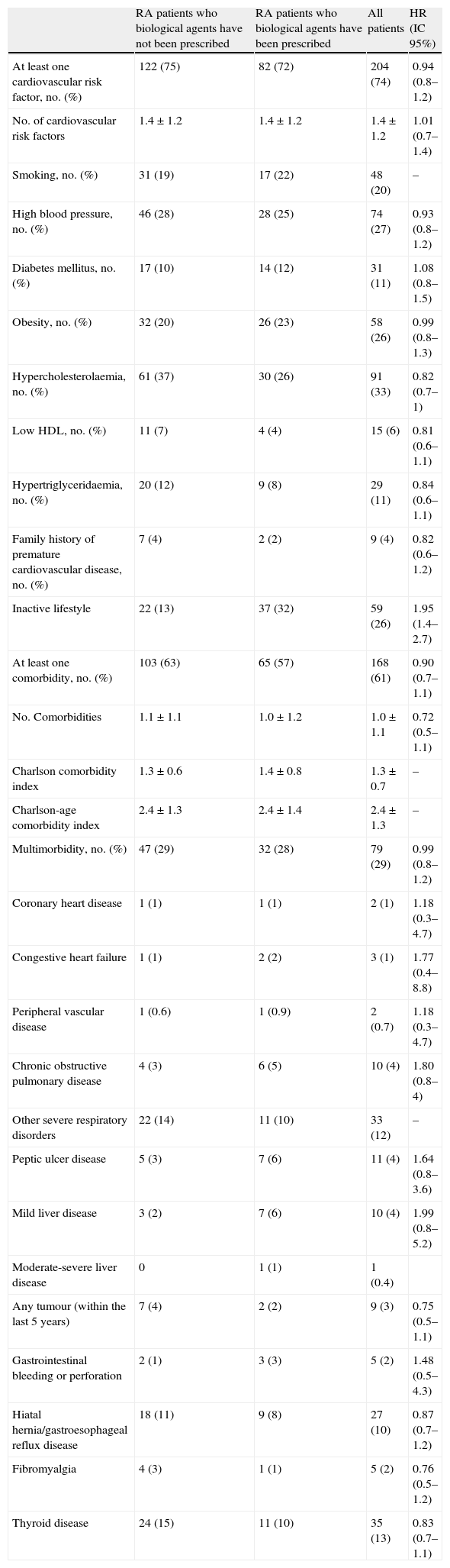
Comorbidities related to rheumatoid arthritisTable 2 displays the percentage of patients who reported different cardiovascular factors and comorbidities at the time of the study. Cardiovascular risk factors and comorbidity frequency were similar between cases and controls. Three-quarters of the patients exhibited certain traditional cardiovascular risk factors. The most prevalent of these risk factors were hypercholesterolaemia, hypertension, obesity, and an inactive lifestyle. The latter was more frequent among the RA patients who were going to receive a biological agent.
Different traditional cardiovascular factors and comorbidities in cases and controls.
| RA patients who biological agents have not been prescribed | RA patients who biological agents have been prescribed | All patients | HR (IC 95%) | |
| At least one cardiovascular risk factor, no. (%) | 122 (75) | 82 (72) | 204 (74) | 0.94 (0.8–1.2) |
| No. of cardiovascular risk factors | 1.4±1.2 | 1.4±1.2 | 1.4±1.2 | 1.01 (0.7–1.4) |
| Smoking, no. (%) | 31 (19) | 17 (22) | 48 (20) | – |
| High blood pressure, no. (%) | 46 (28) | 28 (25) | 74 (27) | 0.93 (0.8–1.2) |
| Diabetes mellitus, no. (%) | 17 (10) | 14 (12) | 31 (11) | 1.08 (0.8–1.5) |
| Obesity, no. (%) | 32 (20) | 26 (23) | 58 (26) | 0.99 (0.8–1.3) |
| Hypercholesterolaemia, no. (%) | 61 (37) | 30 (26) | 91 (33) | 0.82 (0.7–1) |
| Low HDL, no. (%) | 11 (7) | 4 (4) | 15 (6) | 0.81 (0.6–1.1) |
| Hypertriglyceridaemia, no. (%) | 20 (12) | 9 (8) | 29 (11) | 0.84 (0.6–1.1) |
| Family history of premature cardiovascular disease, no. (%) | 7 (4) | 2 (2) | 9 (4) | 0.82 (0.6–1.2) |
| Inactive lifestyle | 22 (13) | 37 (32) | 59 (26) | 1.95 (1.4–2.7) |
| At least one comorbidity, no. (%) | 103 (63) | 65 (57) | 168 (61) | 0.90 (0.7–1.1) |
| No. Comorbidities | 1.1±1.1 | 1.0±1.2 | 1.0±1.1 | 0.72 (0.5–1.1) |
| Charlson comorbidity index | 1.3±0.6 | 1.4±0.8 | 1.3±0.7 | – |
| Charlson-age comorbidity index | 2.4±1.3 | 2.4±1.4 | 2.4±1.3 | – |
| Multimorbidity, no. (%) | 47 (29) | 32 (28) | 79 (29) | 0.99 (0.8–1.2) |
| Coronary heart disease | 1 (1) | 1 (1) | 2 (1) | 1.18 (0.3–4.7) |
| Congestive heart failure | 1 (1) | 2 (2) | 3 (1) | 1.77 (0.4–8.8) |
| Peripheral vascular disease | 1 (0.6) | 1 (0.9) | 2 (0.7) | 1.18 (0.3–4.7) |
| Chronic obstructive pulmonary disease | 4 (3) | 6 (5) | 10 (4) | 1.80 (0.8–4) |
| Other severe respiratory disorders | 22 (14) | 11 (10) | 33 (12) | – |
| Peptic ulcer disease | 5 (3) | 7 (6) | 11 (4) | 1.64 (0.8–3.6) |
| Mild liver disease | 3 (2) | 7 (6) | 10 (4) | 1.99 (0.8–5.2) |
| Moderate-severe liver disease | 0 | 1 (1) | 1 (0.4) | |
| Any tumour (within the last 5 years) | 7 (4) | 2 (2) | 9 (3) | 0.75 (0.5–1.1) |
| Gastrointestinal bleeding or perforation | 2 (1) | 3 (3) | 5 (2) | 1.48 (0.5–4.3) |
| Hiatal hernia/gastroesophageal reflux disease | 18 (11) | 9 (8) | 27 (10) | 0.87 (0.7–1.2) |
| Fibromyalgia | 4 (3) | 1 (1) | 5 (2) | 0.76 (0.5–1.2) |
| Thyroid disease | 24 (15) | 11 (10) | 35 (13) | 0.83 (0.7–1.1) |
HDL, high density lipoproteins; RA, rheumatoid arthritis; DMARDs, disease-modifying antirheumatic drugs.
One patient may have several comorbid conditions.
The values for continuous variables are reported as the means±SD.
Of RA patients, 60% exhibited at least one comorbidity, but only 79 (29%) had multimorbidity. The most prevalent concomitant diseases were hypertension, obesity, and respiratory, thyroid, and upper gastrointestinal disorders. Diabetes mellitus affected 11% of patients, and the incidence of cardiovascular disease was low. There were no cases of dementia, stroke, haematologic malignancies, or acquired immunodeficiency syndrome.
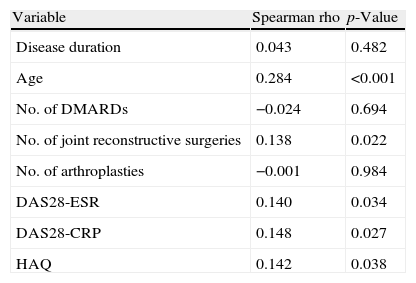
Associated variables with comorbiditySpearman correlation analyses indicated a mild to moderate correlation between the Charlson index and age but moderate correlations between the Charlson index and the number of joint reconstructive surgeries, DAS28-ESR, DAS28-CRP, and HAQ scores (Table 3).
Bivariate Spearman correlation of Charlson comorbidity index with variables of RA (all patients).
| Variable | Spearman rho | p-Value |
| Disease duration | 0.043 | 0.482 |
| Age | 0.284 | <0.001 |
| No. of DMARDs | −0.024 | 0.694 |
| No. of joint reconstructive surgeries | 0.138 | 0.022 |
| No. of arthroplasties | −0.001 | 0.984 |
| DAS28-ESR | 0.140 | 0.034 |
| DAS28-CRP | 0.148 | 0.027 |
| HAQ | 0.142 | 0.038 |
RA, rheumatoid arthritis; DAS28-ESR, 28-joint disease activity – erythrocyte sedimentation rate; DAS28-CRP, 28-joint disease activity – C reactive protein; HAQ, Spanish version of the Health Assessment Questionnaire; DMARDs, disease-modifying antirheumatic drugs.
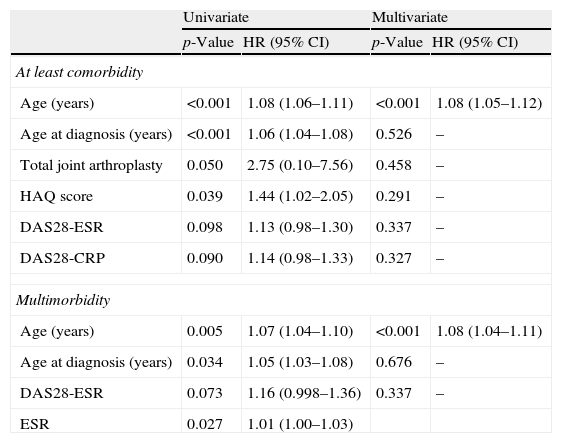
The patients with comorbidities were older, were diagnosed with RA later, had more joint replacements, and had worse HAQ scores (Table 4). Moreover, they tended to have more disease activity at the time of the study. However, other demographic or disease characteristics were not observed. Although men had a higher Charlson index than did women [1.6 (0.8) vs. 1.3 (0.7); Mann–Whitney U-test, p=0.006], this difference disappeared when adjusted for age (Mann–Whitney U-test, p=0.196). Similarly, the patients with multimorbidity were older, were diagnosed with RA later, and exhibited a higher ESR at the time of the study. When the binary log regression model was applied to study the association with both comorbidity and multimorbidity, only patient age remained significantly associated (Table 4). As shown in Table 4, the risk of comorbidity and multimorbidity increases by 8% yearly.
Baseline predictive variables of comorbidity and multimorbidity in RA patients (logistic regression model).
| Univariate | Multivariate | |||
| p-Value | HR (95% CI) | p-Value | HR (95% CI) | |
| At least comorbidity | ||||
| Age (years) | <0.001 | 1.08 (1.06–1.11) | <0.001 | 1.08 (1.05–1.12) |
| Age at diagnosis (years) | <0.001 | 1.06 (1.04–1.08) | 0.526 | – |
| Total joint arthroplasty | 0.050 | 2.75 (0.10–7.56) | 0.458 | – |
| HAQ score | 0.039 | 1.44 (1.02–2.05) | 0.291 | – |
| DAS28-ESR | 0.098 | 1.13 (0.98–1.30) | 0.337 | – |
| DAS28-CRP | 0.090 | 1.14 (0.98–1.33) | 0.327 | – |
| Multimorbidity | ||||
| Age (years) | 0.005 | 1.07 (1.04–1.10) | <0.001 | 1.08 (1.04–1.11) |
| Age at diagnosis (years) | 0.034 | 1.05 (1.03–1.08) | 0.676 | – |
| DAS28-ESR | 0.073 | 1.16 (0.998–1.36) | 0.337 | – |
| ESR | 0.027 | 1.01 (1.00–1.03) | ||
HR, hazard ratio; CI, confidence interval; RA, rheumatoid arthritis; DAS28-ESR, 28-joint disease activity – erythrocyte sedimentation rate; DAS28-CRP, 28-joint disease activity – C reactive protein; HAQ, Spanish version of the Health Assessment Questionnaire; DMARDs, disease-modifying antirheumatic drugs.
We have analysed and compared the relative frequency of comorbidity and multimorbidity in two sex- and age-matched populations of RA patients. Our study group comprised RA patients treated with biological therapy, and our control group comprised RA patients treated with DMARDs alone. Together, these subjects constitute a representative sample of patients with RA, and their clinical and epidemiological characteristics are similar to those found in most large hospital series22 or biological registries.23
RA patients who are prescribed biological agents often have a severe form of the disease and a worse clinical course.24 The observations of our study group support this finding. We found that patients who were prescribed biological agents had higher HAQ scores, had previously received more DMARDs, used more glucocorticoids, and had a higher frequency of total joint arthroplasties than the patients in the control group. Although the elevation of the DAS28 could be explained by the manner in which the patients were selected, a mean baseline DAS28 of 6 indicates severe disease. Despite baseline differences, our study indicates that the relative frequency of comorbidities was similar in the patients who were prescribed biological therapy and those RA patients who were prescribed only DMARDs. This lack of association between inflammatory severity and comorbidity has been observed previously.25 These authors studied a community-based cohort of 183 patients in the early stages of RA, and they observed that measures of inflammation at diagnosis or during follow-up were not predictive of the development of cardiovascular disease. Moreover, they also observed that the vast majority of all comorbidities occurred more than 10 years after the onset of RA, whereas the mean duration of arthritis in our cohort was less than 10 years. Although our univariate analyses revealed that activity factors were associated with comorbidity and multimorbidity, our multivariate analyses revealed no such association. However, this discrepancy may be due to the small sample size or may be because the activity we measured (DAS28, ESR, and CRP) did not account for the accumulated activity burden over time.
Although comorbidity levels in patients treated with biological agents have been studied previously,7 ours is the only study with a control for sex and age. Information regarding the association between the biological treatment of RA and the comorbidity profile of patients is medically relevant and can be of prognostic value. Additionally, the more comorbid illnesses one has, the greater is the interference with treatment, leading to greater medical costs, disability and risk of mortality.4 The most frequent comorbidities in our patients were hypertension, obesity, diabetes, and respiratory, thyroid, and upper gastrointestinal disorders. The relative frequencies of these comorbidities are equivalent to those of other studies. Hypercholesterolaemia was the most prevalent cardiovascular risk factor and exhibited a negative association with the need for biological therapy. Although this difference did not reach statistical significance, higher disease activity is associated with lower total cholesterol levels26 and significantly depressed high-density lipoprotein levels, leading to a higher atherogenic index.27
A limitation of our study is its retrospective design. In a case-controlled study such as ours, the investigator must work backwards. Most comorbidity studies use retrospective cohorts and cross-sectional studies in which data concerning comorbid illnesses are retrieved from patient self-reporting or their medical records. We used data reported by patients and data obtained from medical records. Moreover, both groups are comparable because they were selected consecutively and were matched with respect to the two variables with the greatest impact on the burden of comorbidity, age, and sex.28
Our results are consistent with previous studies and show that the presence of comorbidities and cardiovascular risk factors affects the majority of patients with RA regardless of biological therapy.6,7,29–31 It is well known that in comparison with other chronic diseases, patients with RA suffer a disproportionate comorbidity burden. Similarly high comorbidity is associated with osteoarthritis,6,32 but high comorbidity is not associated with all rheumatic diseases.33 However, the methods used by these studies vary greatly and therefore, the results are difficult to compare. One of the main differences between studies involves the types of diseases and/or disorders included as comorbidities. Associated conditions are diverse and can range from a simple cataract to terminal cancer. We mainly considered the conditions listed in the Charlson index21 (based on 17 diagnoses, each weighted by mortality risks) extended with a few chronic diseases that emphasise measures of impairment and disability. In a disease such as RA, it is important to consider the comorbidities related to mortality and disability outcomes.34
Considerable evidence indicates that patients with RA are at greater risk for developing coronary heart disease.35–37 Although we acknowledge that there may be an underestimation, only 2% of our patients had cardiovascular disease. This figure is lower than that observed by Gabriel et al.6 but the patients in their study were 10 years older because the study included three prevalence cohorts (from 1965) assembled with RA patients that were greater than 63 years old. However, cardiovascular disease is less common in women under 60 years, and our sample consisted of 80% women, 65% of whom were under 60 years. The prevalence of cardiovascular disease in our study is comparable with the latest reports on RA patients treated with biological drugs in this age group.38
Although we know that the risk of multimorbidity in RA is high, we do not understand what heightens this risk. It is possible that RA and its comorbid conditions share the same risk factors. For example, smoking increases the risk of cardiovascular diseases, cancer, and RA. In our study, we identified four factors associated with comorbidity in the univariate analysis, two related to age and the rest related to the severity of RA. However, age was the only predictive factor for both comorbidity and multimorbidity in the multivariate analyses. Because many health problems are known to increase with age, this demographic trend may lead to an increase in the absolute number of chronic conditions in RA patients. For this reason, age always appears as the strongest predictor of comorbidity in addition to pre-existing comorbidity in RA patients.25,39,40
In conclusion, RA patients who are prescribed biological drugs exhibit a comorbidity burden equivalent to those treated with DMARDs only. Age is the main predictive factor of comorbidity in these patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflicts of interestDr. Antonio Fernández Nebro: papers and advice for ABBOT, MSD, PFIZER, ROCHE.