To evaluate the adherence to treatment with Tofacitinib in patients with Rheumatoid Arthritis (RA) using two versions of the self-questionnaire Compliance Questionnaire Rheumatology, CQR19 and CQR5, to determine the variables associated with adherence to Tofacitinib and to compare the performance of both questionnaires.
Material and methodsA cross-sectional study was carried out. We included patients ≥18 years old, with RA (ACR/EULAR criteria 2010) under treatment with Tofacitinib. Sociodemographic data, clinical characteristics, treatment and data on patient evaluation. All the patients completed self-questionnaires CQR19 and CQR5. Statistical analysis: Descriptive statistics. t-Test or Mann Whitney to compare the continuous variables, Chi2 test or Fisher's exact test for the categorical ones. Kappa concordance index. Multiple logistic regression.
ResultsWe included 52 patients, 82.7% women, with a median (m) age of 57.7 years, disease duration m 16 years, 63.5% had comorbidities. Of the patients, 86.5% were treated with Tofacitinib (5 mg BID) and 48% received Tofacitinib as monotherapy. The median time of Tofacitinib treatment was 13 months, 42.3% suspended treatment, and only one patient permanently stopped treatment due to lack of provision. Median CQR19 was 89.5% and 84.6% had an adherence ≥ 80%. The variables significantly associated with adherence ≥ 80% were the presence of comorbidities (p = .014) and older age (p = .033). Considering the CQR5, a similar percentage of patients (82.7%) were adherents to treatment, however, the concordance with CQR19 was low. In the multivariate analysis, older age was the only variable independently associated with good adherence to treatment.
ConclusionsTreatment adherence to Tofacitinib was very good for both presentations. Older age was associated with higher adherence. The agreement between the questionnaires CQR19 and CQR5 was low.
Evaluar la adherencia al tratamiento con Tofacitinib en pacientes con Artritis Reumatoidea (AR) mediante las dos versiones del autocuestionario Compliance Questionnaire Rheumatology, CQR19 y CQR5, determinar las variables asociadas a la adherencia a Tofacitinib y comparar el rendimiento de ambos cuestionarios.
Material y métodosEstudio de corte transversal. Se incluyeron pacientes ≥18 años de edad, con AR (ACR/EULAR 2010) en tratamiento con Tofacitinib. Se consignaron datos sociodemográficos, características clínicas de la enfermedad, tratamientos y datos sobre la evaluación de los pacientes. Todos los pacientes completaron los autocuestionarios CQR19 y CQR5. Análisis estadístico: Estadística descriptiva. T-test o Mann Whitney para variables continuas y test de Chi2 o test exacto de Fisher para las categóricas. Índice de concordancia Kappa. Regresión logística múltiple.
ResultadosSe incluyeron 52 pacientes, 82,7% mujeres, con una edad mediana (m) de 57,7 años, tiempo de evolución de la enfermedad m 16 años. 63,5% presentaban comorbilidades. El 86,5% de los pacientes estaban tratados con Tofacitinib (5 mg dos veces /día) y el 48% recibía Tofacitinib en monoterapia. El tiempo m de tratamiento con Tofacitinib fue de 13 meses. 42,3% suspendieron el tratamiento y un sólo paciente suspendió definitivamente por falta de provisión. La m de CQR19 fue de 89,5% y 84,6% de los pacientes presentaron adherencia ≥ al 80%. Las variables significativamente asociadas con adherencia ≥80%, fueron la presencia de comorbilidades (p = 0,014) y mayor edad (p = 0,033). Considerando el CQR5, un porcentaje similar de pacientes (82,7%) fueron adherentes al tratamiento, sin embargo, la concordancia con CQR19 fue baja (¿: 0,227). En el análisis multivariado, mayor edad fue la única variable independientemente asociada a buena adherencia al tratamiento (p = 0,037).
ConclusionesLa adherencia al tratamiento con Tofacitinib, en ambas formulaciones, fue muy buena. Mayor edad se asoció con mejor adherencia al tratamiento. La concordancia entre los cuestionarios CQR19 y CQR5 fue baja.
Rheumatoid arthritis (RA) is a systemic autoimmune disease which, if not treated promptly and effectively, leads to progressive functional changes resulting in functional disability and this has a major psychosocial and financial impact on the people suffering from it.1 There are currently several existing therapeutic strategies, such as conventional disease modifying anti-rheumatic drugs [c-DMARD], biological [b-DMARD]) and target synthetic [ts-DMARD]) drugs such as tofacitinib, baricitinib and upadacitinib. These small molecules are innovative treatments, due to their mechanism of action, oral administration route and short mean life.
Tofacitinib selectively and competitively inhibits the pathway of the Janus kinase (Jak) 1 and 3, and has demonstrated efficacy and safety for the treatment of patients with moderate to severe RA through its extensive development programme.2 In Argentina two presentations exist, of 5 mg twice daily and 11 mg once a day, both in monotherapy and in combination with c-DMARD.3–5 As far as we know, there are no studies yet which assess the therapeutic adherence of this innovative oral medication.
We are aware that adherence to the pharmacological therapeutic regime of patients with RA generally varies between 30% and 80%, depending on the definition of adherence and the methodology used to measure it.6 By adherence we understand compliance and persistence (regularity and continuity) in taking prescribed medication.7–9 The lack of adherence to pharmacological treatment may lead to more complex diagnostic procedures, a worsening of the disease, increase in disability with the consequent loss of employment, a greater presence of comorbidities and major direct and indirect financial losses within the healthcare system.10 Adherence to medication may be affected by several factors. The World Health Organisation identified five reason for non-adherence to treatment: socioeconomic causes (the cost of the medication), medical care team-related (lack of knowledge and expertise of the medical care professionals), disease-related (disability from the disease), therapy-related (complexity of the medication regime) and patient-related (patient perceptions and expectations).11,12
At present there are several methods which enable adherence to medical treatment to take place. A simple method is through self-referral of the patients through questionnaires. The most highly used and well-known questionnaire is the Compliance Questionnaire on Rheumatology (CQR), which helps to identify factors that contribute to therapy compliance. There are two versions of this questionnaire. CQR19 presents with good reliability and validity of construct, with 87% sensitivity and 67% specificity to detect optimum adherence.13 The other version, the CQR5, is simpler and classifies patients as adherent or non-adherent, according to a mathematical calculation.14
The aims of this study were to: assess the adherence to treatment with tofacitinib in patients with RA using the self-completion questionnaires CQR19 and CQR5, determine the variables associated with it, and compare the performance of both questionnaires.
Material and methodsA cross-sectional study was conducted which consecutively included patients ≥18 years of age with RA according to the ACR-EULAR 201015 criteria, from the outpatient unit, from April to September 2018, who had received treatment with tofacitinib in both presentations. Data collection was made by reviewing medical records and an open interview with the physician treating the patient. Sociodemographic data were collected (age, sex, level of education, civil status, co-habitants, social coverage), disease evolution, time of delayed diagnosis of RA, clinical type (erosive/non erosive), and the presence of the rheumatoid factor (RF) and of anticyclic citrullinated peptide antibodies (anti-CCP). Previous treatments to tofacitinib such as steroids, c-DMARD and/or b-DMARD were recorded.
Regarding tofacitinib, the date of treatment initiation was recorded, together with dose used (5 mg tablets twice a day or the slow-release formula in 11 mg tablets once a day), and concomitant treatments: c-DMARD, NSAIDS and corticotherapy equivalent to prednisone divided into low doses of <10 mg or high doses of ≥10 mg. We also recorded the presentation of adverse events related to the use of tofacitinib, such as allergies, infections, neoplasms, dyslipidaemia, anaemia, neutropenia, increase of transaminases and gastrointestinal events such as digestive intolerance. If the tofacitinib was discontinued, the reasons for the same were classified into: ineffectiveness, adverse events, lack of provision by the healthcare system, patient’s decision and/or surgical requirements. For a complete patient assessment and adherence clinical data were collected from their last visit, the presence of morning stiffness (in minutes), pain and overall activity of the disease according to the patient and to the physician using a visual numerical scale (VNS). Joint counting was carried out of 28 swollen and painful joints,16 as applicable, and levels of acute phase reactants, erythrocyte sedimentation rate (ESR) in mm/h and reactive C protein (RCP) in mg/l. The Health Assessment Questionnaire-Argentinean version (HAQ-A)17 was used to determine functional capacity and the following composite indices were calculated: Disease Activity Score-28 (DAS28)18, Clinical Disease Activity Index (CDAI)19 and Simple Disease Activity Index (SDAI)20 for disease activity.
In order to assess adherence to treatment with tofacitinib, all patients responded to two questionnaires: the Spanish language version of the CQR1913,21 validated in Argentina and the CQR5.14 The CQR19 consists of 19 items, in which the patients indicated their level of agreement regarding certain statements on a 4-point Likert scale (strongly disagree: 1 point; somewhat agree: 2 points; somewhat agree: 3 points; strongly agree: 4 points). Six items present negative statements (number 4, 8, 9, 11, 12 and 19) and, as a consequence, the scoring has to be inverted (4 = 1, 3 = 2, 2 = 3, 1 = 4). Calculation of scoring arises from the sum of the points of all items, deducting 19 and then dividing them by .57, obtaining an adherence scale which varied from 0 (no adherence) to 100 (perfect adherence).13 The CQR5 consists of 5 items, maintaining questions numbers 2, 3, 5, 6 and 17 of the CQR19. Response is the same using the same Likert scale from 1 to 4. After these two formulae are applied (one of low adherence and the other of high adherence) and the results are compared. If the result of the high adherence is higher than that of the low adherence, the patient is classified as being of high adherence (≥80%), and if the low adherence formula is higher than that of the high one, they are considered to be of low adherence (<80%).14 The study was presented at the Argentinean Rheumatology Congress, in Mendoza in 2018. Following this, for publication, the data were reviewed and updated in relation to new adverse events or possible treatment discontinuation.
Statistical analysisDescriptive statistics were used. The categorical variables were expressed in frequencies and percentages and the continuous ones as a median (m) and interquartile range (IQR), of mean X¯ and standard deviation (SD), depending on their distribution. Comparison of continuous variables was made using the Student’s t-test or the Mann Whitney test and ANOVA, as applicable and the comparison of the categorical variables was made using the Chi-squared test or the exact Fisher test. The kappa test was using to assess consistency between questionnaires CQR19 and CQR5. Multiple logistic regression was used to determine the variables associated with adherence, using adherence measured by CQR19 ≥80% as the dependent variable and with a p < .1 in the univariate as independent variables. Significance was considered to be p ≤ .05.
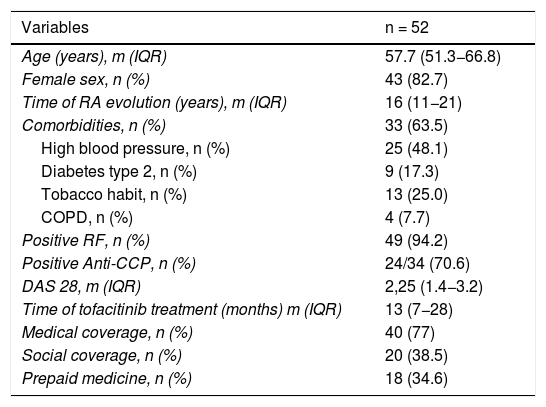
ResultsFifty two patients were included, of whom 82.7% (43) were women, with a mean age (m) of 57.7 years (IQR: 51,3–66,8) and a mean time of disease evolution of 16 years (IQR: 11−21). Forty patients (77%) had medical coverage. Regarding the disease characteristics, 49 patients (94.2%) were seropositive for the RF, whilst 34 (70.6%) were anti-CCP positive. Forty five patients (86.5%) presented with radiographic erosions. Sixty-three point five per cent presented with comorbidities, the most common being high blood pressure (39%) and a tobacco habit (27%). DAS28 m 2.25 (IQR: 1.39–3.25). The other population characteristics are contained in Table 1. Eighteen patients received tofacitinib due to inadequate response (IR) to c-DMARD and 35 (67.3%) due to IR to b-DMARDs; 18 of them then for failure to 1st b-DMARD, 10 then for failure to 2nd b-DMARD, 5 then for failure to 3rd b-DMARD and 2 patients then for failure to 4th b-DMARD. The biologic agents previously received were: anti-TNF 32 agents, 15 abatacept, 10 tocilizumab and 4 rituximab. A total of 45 (86.5%) patients treated with tofacitinib used the 5 mg dose twice a day, whilst 8 (13.4%) used the formulation of 11 mg/day. Almost half of the patients (48%) received tofacitinib in monotherapy. Of the patients in combined treatment, 17 (63%) received methotrexate, 9 (33.3%) leflunomide and one patient sulphasalizine. Median time of treatment with tofacitinib was 13 months (IQR: 7−28).
Sociodemographic, medical and therapeutic characteristics of the population under study.
| Variables | n = 52 |
|---|---|
| Age (years), m (IQR) | 57.7 (51.3−66.8) |
| Female sex, n (%) | 43 (82.7) |
| Time of RA evolution (years), m (IQR) | 16 (11−21) |
| Comorbidities, n (%) | 33 (63.5) |
| High blood pressure, n (%) | 25 (48.1) |
| Diabetes type 2, n (%) | 9 (17.3) |
| Tobacco habit, n (%) | 13 (25.0) |
| COPD, n (%) | 4 (7.7) |
| Positive RF, n (%) | 49 (94.2) |
| Positive Anti-CCP, n (%) | 24/34 (70.6) |
| DAS 28, m (IQR) | 2,25 (1.4−3.2) |
| Time of tofacitinib treatment (months) m (IQR) | 13 (7−28) |
| Medical coverage, n (%) | 40 (77) |
| Social coverage, n (%) | 20 (38.5) |
| Prepaid medicine, n (%) | 18 (34.6) |
Anti-CCP: Anticyclic citrullinated peptide antibodies; COPD: Chronic obstructive pulmonary disease; IQR: Interquartile range; m: Median; RF: Rheumatoid facture.
Twenty-two patients (42.3%) suspended their treatment with tofacitinib: 21 temporarily and only one patient definitively, prior to the data analysis for this publication. The reasons for discontinuation were infections (10: 4 cases of zoster herpes [HZ], 4 lower urinary tract infections [UTI] and 2 community-acquired pneumonias [CAP]); the other causes were planned surgery in 7 patients and lack of provision in 4 patients. Most of the events were treated in the outpatient’s unit and were resolved without any complications. The only patient who discontinued treatment definitively did so due to the lack of provision of tofacitinib by the healthcare system.
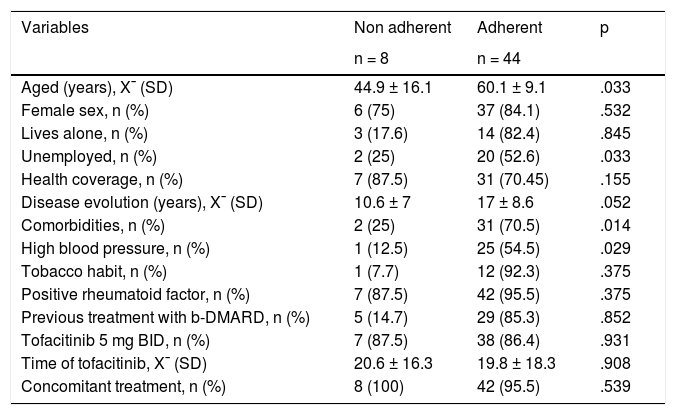
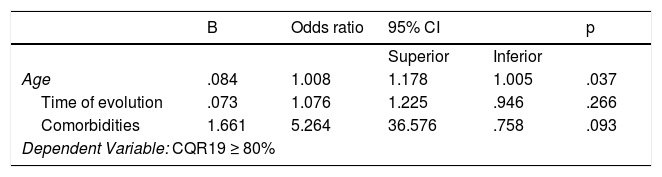
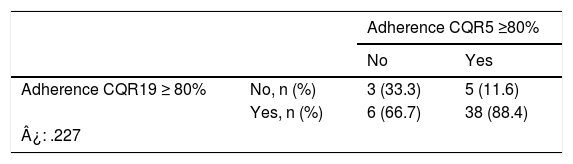
Regarding adherence to tofacitinib, the result of the CQR19 was m 89.5% (IQR: 84.2–94.3). Forty four patients (84.6%) presented with an adherence ≥80%, and this was significantly associated with the presence of comorbidities (70.5% vs 29.5%; p = .014) and older age (X¯ 60 ± 9 years vs X¯ 45 ± 6 years; p = .033) (Table 2). In the multivariate analysis, using CQR19 ≥80% as the dependent variable, older age remained separately associated from higher treatment adherence (OR: .09; 95% CI: 1.06–1.18; p = .037) (Table 3). When we assessed the results of the CQR5, 82.7% of patients had adhered to treatment. Consistency between both questionnaires, CQR19 and CQR5, was low (¿: .227) (Table 4).
Variables associated with the presence or absence of adherence (CQR19 ≥ 80%).
| Variables | Non adherent | Adherent | p |
|---|---|---|---|
| n = 8 | n = 44 | ||
| Aged (years), X¯ (SD) | 44.9 ± 16.1 | 60.1 ± 9.1 | .033 |
| Female sex, n (%) | 6 (75) | 37 (84.1) | .532 |
| Lives alone, n (%) | 3 (17.6) | 14 (82.4) | .845 |
| Unemployed, n (%) | 2 (25) | 20 (52.6) | .033 |
| Health coverage, n (%) | 7 (87.5) | 31 (70.45) | .155 |
| Disease evolution (years), X¯ (SD) | 10.6 ± 7 | 17 ± 8.6 | .052 |
| Comorbidities, n (%) | 2 (25) | 31 (70.5) | .014 |
| High blood pressure, n (%) | 1 (12.5) | 25 (54.5) | .029 |
| Tobacco habit, n (%) | 1 (7.7) | 12 (92.3) | .375 |
| Positive rheumatoid factor, n (%) | 7 (87.5) | 42 (95.5) | .375 |
| Previous treatment with b-DMARD, n (%) | 5 (14.7) | 29 (85.3) | .852 |
| Tofacitinib 5 mg BID, n (%) | 7 (87.5) | 38 (86.4) | .931 |
| Time of tofacitinib, X¯ (SD) | 20.6 ± 16.3 | 19.8 ± 18.3 | .908 |
| Concomitant treatment, n (%) | 8 (100) | 42 (95.5) | .539 |
B-DMARDs: Biological-Disease Modifying Anti-Rheumatic Drugs; BID: twice a day; SD: Standard Deviation; X¯: Mean.
Variables associated with adherence to treatment with tofacitinib in patients with rheumatoid arthritis according to CQR19 (≥80%). Multiple logistic regression.
| B | Odds ratio | 95% CI | p | ||
|---|---|---|---|---|---|
| Superior | Inferior | ||||
| Age | .084 | 1.008 | 1.178 | 1.005 | .037 |
| Time of evolution | .073 | 1.076 | 1.225 | .946 | .266 |
| Comorbidities | 1.661 | 5.264 | 36.576 | .758 | .093 |
| Dependent Variable: CQR19 ≥ 80% | |||||
CI: confidence interval; CQR: Compliance Questionnaire Rheumatology 19.
In this study we assessed treatment adherence to pharmacological treatment with tofacitinib and we observed that 84.6% of the patients had ≥ 80% adherence measured by the CQR19 and 82.7% by the CQR5. As far as we are aware, this is the first study to directly assess treatment adherence of a small molecule in RA patients. A recently published retrospective study compared the persistence and adherence to vedolizumab in patients with inflammatory bowel disease (IBD) and to tofacitinib in patients with RA, using infliximab as the adherence comparer between the two disease. After indirect adjustment for each disease, adherence was significantly greater for treatment with endovenous infusión.22 This study had certain limitations due to its retrospective methodology and to indirect comparison between two different diseases which suggest results should be treated with caution.
Some reports both in RA and in other disease show greater adherence to the endovenous route compared with oral and subcutaneous routes.23 Stolshek et al.24 observed greater adherence to patients treated with infliximab compared with patients treated with other subcutaneous b-DMARD. These events could be due to the fact that endovenous treatment requires the need to use an infusion centre for treatment, in most cases with a previous appointment, and this situation could positively impact patient adherence.
In a systematic review of the literature, adherence in RA patients varied between 30% and 80% depending on the type of drugs prescribed, the established dose, follow-up time, disease evolution time and methods used to assess it. This variability in adherence could also be due to the studies included in this review assessing c-DMARD and b-DMARD, whilst in others NSAIDS c-DMARD25,26 were included.
Grijalva et al.27 made a retrospective study of treatment adherence to c-DMARD (methotrexate, leflunomide and sulfasalazine) and to b-DMARD (infliximab, etanercept and adalimumab) in patients with RA using the medication possession rate (MPR), detecting greater adherence to biologic agents. This is a centralized dispensing of medicines method in hospital pharmacies. The number of days a patient possesses medication is calculated, divided by the number of days of treatment. These authors observed higher adherence to b-DMARD in monotherapy compared to combined therapies and with c-DMARD. This higher adherence to the b-DMARD vs c-DMARD could not only be conditioned by administration route but also by a greater convenience for the patients during intervals of more prolonged doses.
In our centre, in 118 patients with RA, adherence to methotrexate measured through CQR5 was 86.6%28 and b-DMARD was 78%,29 demonstrating good adherence for both types of drugs and similar adherence reported for tofacitinib to this study. In contrast, other studies from Argentina and Australia found levels of adherence to treatment with c-DMARD and b-DMARD <70%.30–32 These differences could be due to socioeconomic, cultural, ethnic and psychological factors.
One of the aspects that could have a positive impact on adherence is the perception of the disease.31,33 Wabe et al.31 observed that belief in the need for medication, self-sufficiency and patients of an older age were associated to greater adherence. This association coincides with the results of our study, since older patients presented with significantly higher treatment adherence. This could be due to the fact that these patients have a better perception of the disease and therefore consider the need for therapy compliance. Although we do not measure the perception of the disease in our patients, Hughes et al.34 found there was greater perception of the disease in older patients, who presented with greater disability measured by the HAQ. In turn, Park et al.35 detected that older adult patients committed fewer efforts in compliance of the therapeutic regimes compared with middle aged adults. This situation could be due to the faster pace of life younger patients had, which had a negative impact on adherence.
Treatment discontinuation in our study was high, at 42.3%, although, with the exception of one case, it was temporary in all patients. The most commonly given reason was infections and in all cases these were resolved with no complications. Regarding reasons for discontinuation, a multicentre study of the real life of 419 patients with RA from 9 Latin American countries, the frequency of definitive discontinuation of tofacitinib was 13.4%: 6.9% due to lack of efficacy, 3.6% due to adverse events, and 2.9% for other reasons.36 The frequency of definitive discontinuation of the treatment in our study was low. A total of 5 patients had to discontinue due to lack of provision by the healthcare system. Of them, only one was definitively discontinued.
In our analysis we observed low consistency between the two versions of the CQR. This could be due to the fact that the CQR19 has a simpler quantification method and presents with a better construct validity.13,21 Furthermore, one difference to emphasize between both is that the CQR19 determines the percentage of adherence, whilst the CQR5 dichotomizes patients between adherents and non-adherents.14 In the literature we did not find a study that assessed consistency between both questionnaires, although it was reported that the CQR5 has a 97% specificity on identifying the high adherents classified by the CQR19 and 69% sensitivity identifying the low adherents.14
It is important to mention the limitations of this study: the number of patients analysed was relatively low and the study was cross-sectional, which meant that no long-term assessment of adherence to treatment with tofacitinib could be made, and it would be of interest to follow this up in future studies. Finally, measuring compliance to tofacitinib was self-referral, and could be subject to recall bias.
ConclusionsThis study showed a particularly good self-referral adherence to treatment with tofacitinib. Older age was independently associated with higher adherence. There was low consistency between the two versions of the CQR.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Barbich T, Cerda OL, Schneeberger EE, Citera G. Adherencia al tratamiento con tofacitinib en pacientes con artritis reumatoide en la práctica clínica diaria. Reumatol Clin. 2022;18:164–168.