Atlas of Axial Spondyloarthritis in Spain 2017 aims to better understand the reality of the patients suffering from this disease from an integrated approach.
MethodsThe Atlas 2017 based its results on an extensive cross-sectional patient survey conducted in Spain (2016), validated by a multidisciplinary group of experts on spondyloarthritis.
ResultsData from 680 patients with axSpA were obtained, most of them suffered from AS, were HLA-B27 positive, older than 45 years, and live as part of a couple. A large percentage had university studies, were disabled and members of a patient association. Patients reported a diagnostic delay of 8.5 years, high disease activity (BASDAI 5.5±2.2), moderate-important stiffness (61.0%), medium-high functional limitation (74.9%), and psychological distress (GHQ 5.7±4.5). A total of 54.7% reported taking NSAIDs, 28.4% DMARDs, 36.3% biological therapy and 32.2% were not receiving pharmacological treatment.
ConclusionsThe Atlas survey data reveals still a long diagnostic delay, high disease activity, psychological distress, while an important proportion could be undertreated.
El Atlas de Espondiloartritis Axial en España 2017 tiene como objetivo comprender mejor la realidad de los pacientes que padecen esta enfermedad desde un enfoque integrado.
MétodosEl Atlas 2017 basó sus resultados en una amplia encuesta transversal de pacientes realizada en España (2016), validada por un grupo interdisciplinar de expertos en espondiloartritis.
ResultadosSe obtuvieron datos de 680 pacientes con EspAax. La mayoría de ellos sufría EA, eran HLA-B27 positivo, mayores de 45 años y vivían en pareja. Un gran porcentaje tenía estudios universitarios, discapacidad reconocida y era miembro de una asociación de pacientes. Los pacientes declararon un retraso diagnóstico de 8,5 años, alta actividad de la enfermedad (BASDAI 5,5±2,2), rigidez moderada-importante (61,0%), limitación funcional moderada-alta (74,9%) y problemas psicológicos (GHQ 5,7±4,5). Un total del 54,7% declaró estar tomando AINE, el 28,4% FAME, el 36,3% terapia biológica, mientras que el 32,2% no recibía ningún tipo de tratamiento farmacológico.
ConclusionesLos datos de la encuesta Atlas revelan todavía un enorme retraso diagnóstico, alta actividad de la enfermedad, problemas psicológicos, mientras que una proporción importante de pacientes podrían estar infratratados.
The term Spondyloarthritis (SpA) describes a group of chronic inflammatory diseases that share clinical, pathogenic, genetic, radiological, epidemiological, and therapeutic response characteristics.1 Currently, patients with SpA are commonly classified according to the predominant manifestations in peripheral and axial SpA (axSpA).2,3 At the same time, axSpA includes Ankylosing Spondylitis (AS) and Non-Radiographic Axial Spondyloarthritis (nr-axSpA), which represents a form of axSpA without a substantial radiographic structural damage.
Nowadays, the mean estimated AS prevalence in Europe (from 36 eligible studies) is 23.8 per 10,000.4 Disease onset occurs commonly before 45 years of age, leads to severe functional disability and loss of work capacity, generally coinciding with a productive life period.5 Some studies have shown an unemployment rate of 25% among AS patients; in up to 21% of cases this is attributed to the disease.6 In this sense, one main challenge of axSpA is the huge diagnostic delay, which prevents initiating the most appropriate treatment during the initial phases. This is largely associated with disease progression, irreversible structural damage, and loss of patient mobility.7–9 During the last decade, many advances in the field of axSpA have been achieved. In 2009, the ASAS classification criteria for axSpA were developed, including the implementation of the magnetic resonance imaging of the sacroiliac joints in patients with suspected axSpA.1,10 This results in an increased ability to detect inflammation during the first stages of the disease, much longer before structural damage can be detected by other methods, such as x-ray or computed tomography.11 In addition, new therapies (biological therapies) have been developed and have shown to be efficacious in patients with axSpA too.
In Spain, data available for both diagnosis and disease outcomes are drawn from studies completed more than a decade ago.12,13 Therefore, to date, it is unknown whether or not all the medical advances achieved have had any impact on the course of the disease and quality of life of patients. On the other hand, there is a wealth of information about axSpA from the clinical point of view, in addition to the most appropriate pharmacological treatments. However, few studies have evaluated aspects such as healthcare planning, treatment resources, reasons for diagnostic delay, functional limitations, psychological status, productivity losses, costs associated with disease management, or patient opinion. Thus, it was decided to undertake the Atlas of Axial Spondyloarthritis in Spain 2017 project.
The Atlas 2017 aims to better understand the current reality of people suffering from axSpA from an integrative perspective based on scientific evidence, expert knowledge, and patient opinion. The study objectives were to assess the main barriers and difficulties for diagnosis, health care, pharmacological and non-pharmacological treatments, labor productivity losses, and the direct health and non-medical costs of the Spanish National Health System derived from the disease.
MethodsWorking groupAtlas 2017 is an initiative of the Spanish Coordinator of Patient Associations of Spondylarthritis (CEADE), carried out by the research group Health & Territory Research (HTR) of the University of Seville and the Max Weber Institute, with the collaboration of the Spanish Society of Rheumatology (SER) and sponsored by Novartis Farmacéutica, Spain.
The study included a scientific committee composed of four rheumatologists belonging to the Spanish Group of Spondyloarthritis of the Spanish Society of Rheumatology (GRESSER), two patients with axSpA representing the patient association, two primary care physicians, three health economists, and one health geographer. An advisory committee was also established which consisted of two psychologists, one nurse, one pharmacist, and one physiotherapist. All of the professionals had experience in the clinical care of patients and/or in the development of epidemiological studies. The members of the scientific committee were charged with assessing and validating the study results, as well as making proposals to better understand the current management of axSpA in Spain. In order to do so, the committee met three times over the course of a year. The advisory committee supported the resolution of doubts about specific matters, communicating mainly through the internet and via teleconferences.
SurveyA patient questionnaire was designed for individuals suffering from axSpA based on expert opinion of a panel of rheumatologists and patients with axSpA and a broad literature review to identify relevant aspects related to axSpA in the following areas: diagnosis, treatment, comorbidity, employment, limitations and psychological state from 2000 up until November 2017. Articles were limited to studies in the English or Spanish language. Inclusion criteria were age of 18 years or older (adults), being diagnosed of axSpA, and living in Spain during the survey. The questionnaire, developed over four months (from January to April 2016), was used in a pilot study of a group of 17 axSpA patients belonging to different age groups and with varying educational levels. This sample was personally interviewed in different rounds.
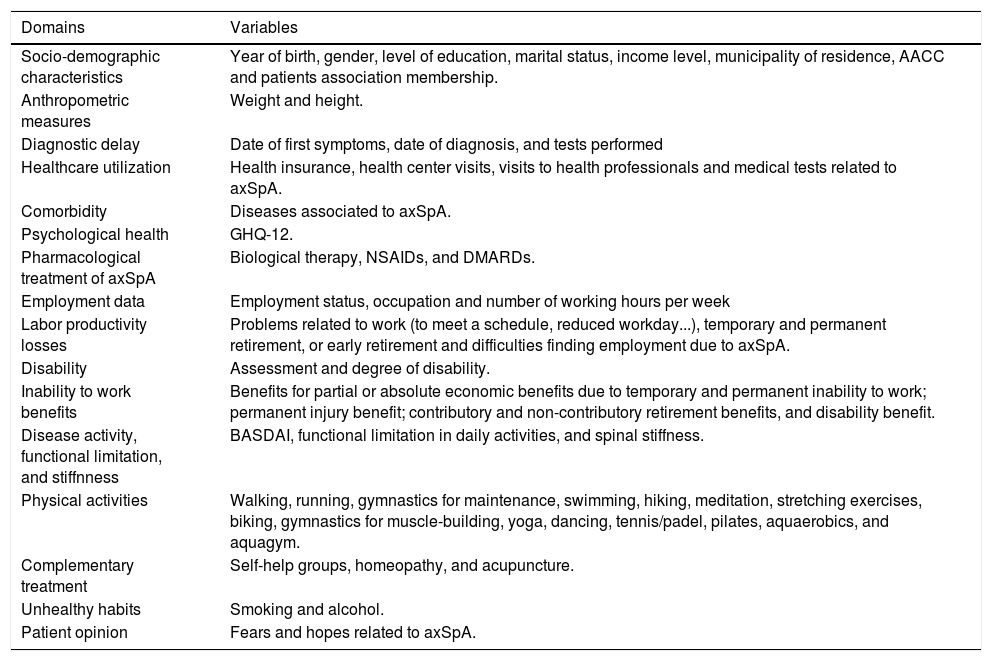
This process allowed for the development of the questionnaire in its final version, which is composed of 116 items and four open-ended qualitative questions that are included in the 16 thematic domains described in Table 1.
Variables included within the patient questionnaire of the Atlas 2017.
| Domains | Variables |
|---|---|
| Socio-demographic characteristics | Year of birth, gender, level of education, marital status, income level, municipality of residence, AACC and patients association membership. |
| Anthropometric measures | Weight and height. |
| Diagnostic delay | Date of first symptoms, date of diagnosis, and tests performed |
| Healthcare utilization | Health insurance, health center visits, visits to health professionals and medical tests related to axSpA. |
| Comorbidity | Diseases associated to axSpA. |
| Psychological health | GHQ-12. |
| Pharmacological treatment of axSpA | Biological therapy, NSAIDs, and DMARDs. |
| Employment data | Employment status, occupation and number of working hours per week |
| Labor productivity losses | Problems related to work (to meet a schedule, reduced workday...), temporary and permanent retirement, or early retirement and difficulties finding employment due to axSpA. |
| Disability | Assessment and degree of disability. |
| Inability to work benefits | Benefits for partial or absolute economic benefits due to temporary and permanent inability to work; permanent injury benefit; contributory and non-contributory retirement benefits, and disability benefit. |
| Disease activity, functional limitation, and stiffnness | BASDAI, functional limitation in daily activities, and spinal stiffness. |
| Physical activities | Walking, running, gymnastics for maintenance, swimming, hiking, meditation, stretching exercises, biking, gymnastics for muscle-building, yoga, dancing, tennis/padel, pilates, aquaerobics, and aquagym. |
| Complementary treatment | Self-help groups, homeopathy, and acupuncture. |
| Unhealthy habits | Smoking and alcohol. |
| Patient opinion | Fears and hopes related to axSpA. |
AACC, Autonomous Community; axSpA, Axial Spondyloarthritis; GHQ-12, General Health Questionnaire – 12 items; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; NSAIDs, nonsteroidal anti-inflammatory drugs; DMARDs, disease-modifying antirheumatic drugs.
The dissemination of the patient questionnaire for the recruitment of a sufficient representative sample size was made through general and specific press releases to the medical community, emails to SpA patient association members, CEADE website, websites of local SpA patient associations, patients’ social networks, and during the I Spanish Annual Meeting of Patients with Spondyloarthritis. A total of 838 patients with axSpA anonymously accessed the online questionnaire between May 1 and August 15, 2016. After the validation and normalization of the information, the sample consisted of a total of 680 patients who responded to the majority of the questionnaire (completion rate was higher than 75%).
Indices and scales for the assessment of disease effectsIn addition to the items included in Table 1, the patients included in the study completed five indices, two of which had been previously validated - the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the General Health Questionnaire (GHQ-12). Three others created specifically for this project were also included: the Spinal Stiffnes Index, the Functional Limitation Index, and the Beneficial Physical Activities Index.
BASDAI: Self-reported disease status was measured using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).14 The BASDAI scale measures disease activity in patients with AS. It can be self-administered and includes six questions pertaining to the following symptoms: fatigue, spinal pain, joint pain/swelling, areas of localized tenderness, and morning stiffness, rated on an analog scale from 0 (no activity) to 10 (maximum activity). This instrument has shown good psychometric properties and applicability to everyday clinical practice.15 In the present study, the Cronbach's alpha score for the BASDAI was 0.879, close to one and above the minimum established levels, which guarantees that this scale is reliable to measurement of the self-reported disease activity.
GHQ-12: developed by Goldberg and Williams in 1988, this questionnaire assesses the severity of psychological distress during the weeks prior to participation, with the objective of detecting psychological morbidity and possible cases of psychiatric disorders.16,17 The validated cut-off point on the GHQ scale for the Spanish version of the questionnaire is ≥3, indicating that individuals with a score of ≥3 may be experiencing psychological distress.18 The GHQ-12 shows good reliability in the various studies carried out with Cronbach's alphas ranging from 0.82 to 0.86.19 A recent study developed in Spain describes a Cronbach's alpha of 0.76.16 In our study, we obtained a Cronbach's alpha of 0.934 which supports the hypothesis of the scale reliability.
Spinal Stiffness Index: Based on the ASAS concept of spinal stiffness which is defined by intensity and duration upon awakening.20 Therefore, one of the questionnaire items aimed at assessing the degree of spinal stiffness experienced by patients, distinguishing between the three spinal regions: cervical, thoracic, and lumbar. The index resulted from adding unweighted degree of rigidity in these three regions on a scale of lesser to greater effect (from 0 to 9): where a value of 0 would imply no stiffness, between 1 and 3 light stiffness, between 4 and 5 moderate stiffness, and between 6 and 9 significant stiffness. In our study we obtained a Cronbach alpha of 0.850, which confirms the reliability of this scale for the assessment of stiffness.
Functional Limitation Index: Generated by adding the non-weighted degree of functional limitation registered for 18 different activities of daily living (dressing, grooming, bathing, tying shoelaces, moving around the home, stairs, getting to/out of bed, toilet, shopping, preparing meals, eating, cleaning, walking, using public transportation, going to the doctor, driving, physical exercise, sexual relations), using a score of 0–3 (0 no limitation, 1 low limitation, 2 medium limitation, and 3 high limitation), with a total result between 0 and 54. Thus, a global functional limitation value between 0 and 18 would imply low limitation, between 18 and 36 medium limitation, and between 36 and 54 high limitation. The Cronbach alpha value obtained for this scale is 0.964, guaranteeing its reliability as an instrument for assessing limitation.
Beneficial Physical Activities Index: Not all physical activities are beneficial for patients with axSpA and, therefore, it was necessary to identify the appropriate activities.21 In particular, physical activities that promote good posture are recommended, as well as extending and rotating the trunk.22 To evaluate the performance of beneficial physical activities, a dichotomous indicator was established including six beneficial physical activities classified (Pilates, meditation, yoga, swimming, aquaerobics, and aquagym) according to expert opinion and the results from the systematic literature review.23–25
The calculation of direct health and non-medical costs was made using the social perspective by multiplying the resources used by each patient with AS, for the unit price of each resource, in the year prior to answer the questionnaire. This data was self-reported by patients. Direct health costs include the AS-related cost of medical visits, medical tests, emergencies, hospital admissions, and medication (pharmacological and administration costs). Direct non-medical costs include alternative treatments for AS in the preceding 12 months (e.g. acupuncture and homeopathy) and expenditures on rehabilitation therapies or physical exercise, indicated directly by patients in monetary value (no including household and car adaptations, nor the out-of-pocket expenses incurred in transportation).
Productivity losses refer to reduced labor productivity related to AS (ability to meet a schedule, reduced working hours, temporary and permanent sick leave, or early retirement). Following the Human Capital approach, a cost has been allocated to working days lost or lost wages. For this, the average wage was assigned according to gender and occupation level; in the case of workers and temporary sick leave, and the average wage according to gender, and average hours worked per year for the other situations that imply productivity loss. The wage data was extracted from the National Statistics Institute of Spain (INE).26
ResultsA total of 838 patients with axSpA accessed the questionnaire. After the validation and homogeneization process, the survey database was comprised of a total of 680 patients, distributed homogeneously by gender through the 17 Spanish Autonomous Communities.
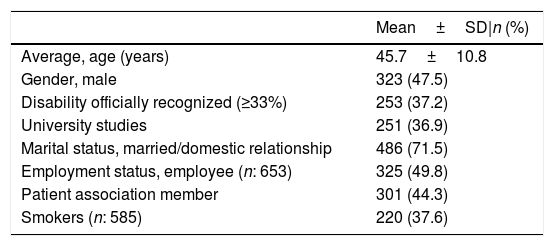
The sociodemographic characteristics of the included patients are detailed in Table 2. Gender distribution was homogeneous across the sample. The majority of patients were married, within the range of working ages, and with a significant percentage having some degree of recognized disability. On the other hand, although an important part of the survey dissemination was carried out through SpA patient associations, less than half of respondents were members of one of these patient associations.
Sociodemographic characteristics of the 680 survey respondents included in the Atlas.
| Mean±SD|n (%) | |
|---|---|
| Average, age (years) | 45.7±10.8 |
| Gender, male | 323 (47.5) |
| Disability officially recognized (≥33%) | 253 (37.2) |
| University studies | 251 (36.9) |
| Marital status, married/domestic relationship | 486 (71.5) |
| Employment status, employee (n: 653) | 325 (49.8) |
| Patient association member | 301 (44.3) |
| Smokers (n: 585) | 220 (37.6) |
N=680, unless otherwise specified.
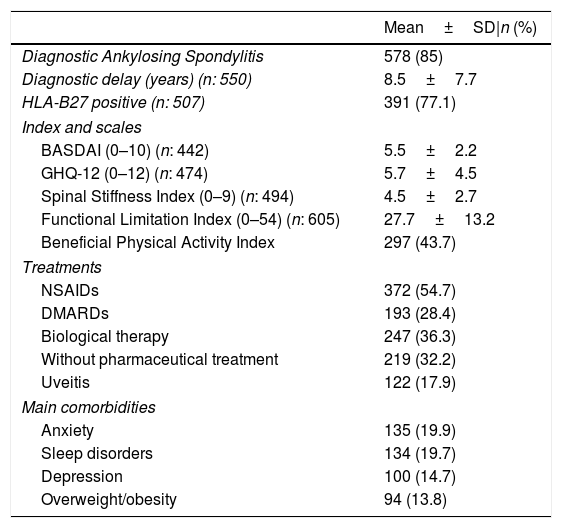
Table 3 shows the clinical self-reported characteristics of patients. In addition to the data shown in this table, it is noteworthy that 61% of patients reported moderate to severe stiffness and 74.9% had a medium-high degree of limitation. On the other hand, 76.7% of the patients presented a BASDAI above the cut-off point for active disease (≥4). Similarly, the GHQ-12 mean score exceeded the cut-off point to define psychological distress (≥3) with 65.4% of the patient above this point,18 with the main comorbidities self-reported being anxiety, sleep disorder, depression and overweight/obesity. Most patients were taking NSAIDs, followed by biological therapy, and DMARDs. However, a significant percentage of patients reported that they were not currently receiving any pharmacological treatment despite the 2016 update of the ASAS-EULAR management recommendations for axSpA.27
Clinical self-reported characteristics of patients included in the Atlas 2017, according to patient responses.
| Mean±SD|n (%) | |
|---|---|
| Diagnostic Ankylosing Spondylitis | 578 (85) |
| Diagnostic delay (years) (n: 550) | 8.5±7.7 |
| HLA-B27 positive (n: 507) | 391 (77.1) |
| Index and scales | |
| BASDAI (0–10) (n: 442) | 5.5±2.2 |
| GHQ-12 (0–12) (n: 474) | 5.7±4.5 |
| Spinal Stiffness Index (0–9) (n: 494) | 4.5±2.7 |
| Functional Limitation Index (0–54) (n: 605) | 27.7±13.2 |
| Beneficial Physical Activity Index | 297 (43.7) |
| Treatments | |
| NSAIDs | 372 (54.7) |
| DMARDs | 193 (28.4) |
| Biological therapy | 247 (36.3) |
| Without pharmaceutical treatment | 219 (32.2) |
| Uveitis | 122 (17.9) |
| Main comorbidities | |
| Anxiety | 135 (19.9) |
| Sleep disorders | 134 (19.7) |
| Depression | 100 (14.7) |
| Overweight/obesity | 94 (13.8) |
N=680, unless otherwise specified; HLA-B27, Human Leukocyte Antigen B27; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; GHQ-12, General Health Questionnaire – 12 items; NSAIDs, nonsteroidal anti-inflammatory drugs; DMARDs, disease-modifying antirheumatic drugs; uveitis, current or antecedent; diagnostic delay, difference between time from symptom onset and time from diagnosis; treatments, last 12 months.
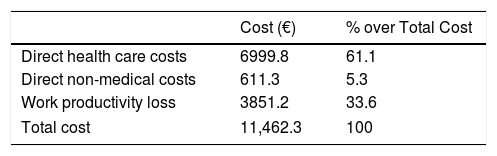
Atlas 2017 also assesses the economic burden of AS in Spain. The social perspective was used and the estimated average cost per patient per year is €11,462.3 (including direct health care costs, direct non-medical costs and productivity losses). Out of this, more than half are related to direct health costs, followed by costs associated with labor productivity loss and, finally, direct non-medical costs (Table 4).
DiscussionAtlas 2017 is the most ambitious project promoted from Spanish Coordinator of Associations of Spondyloarthritis (CEADE) to date and aims to respond to a shortage of information on axSpA in Spain, including the patient's perspective. The data collected provide a comprehensive and current vision of axSpA in Spain and the implications for patients coping with this chronic condition. The main tool of the Atlas has been the anonymous online survey carried out on a wide sample of patients distributed throughout the country, promoted, and disseminated by the CEADE patient associations. However, this was not a limitation since only 44.3% of respondents were members of patient associations.
Among the most relevant data, we should highlight a diagnosis delay of 8.5 years and the large burden associated with the disease in terms of disability. This describes a worrisome situation and highlights the difficulties that still persist today regarding early diagnosis, which could largely prevent its evolution. In addition, less than 50% of respondents were working at the time of the survey, which is of concern considering that the average age was around 45 years. On the other hand, the mean 5.5% BASDAI reported by patients indicates that, for most, the disease is not controlled. Finally, this study also demonstrates the great psychological impact of this disease, since the GHQ-12 showed a high average score (value 5.7). Using this data, a document was created (English and Spanish versions are available at www.espondiloartritisaxial.org/atlas) that deepens the knowledge of axSpA in Spain and provides new evidence aimed at improving patient care and quality of life.
One of the main contributions of the Atlas 2017 has been the incorporation of the patient's perspective. Unlike other reports or research carried out on axSpA, it not only seeks to capture the burden of disease from a medical and health perspective, but also to reflect the patient's overall experience in terms of the physical, social, emotional, labor and economic limitations, among others. This study outline the importance of a strong partnership developed between researchers (academic institutions), scientific societies of rheumatology, healthcare professionals and patients with rheumatic diseases.
In the future, the database derived from the Atlas 2017 survey will allow analysts to answer key research questions, such as: what are the reasons for the diagnostic delay?; what are the key risk factors for structural damage?; how does the disease severity affect psychological distress?; what is the relationship between unhealthy habits and disease activity?; or the possible benefits of belonging to a patient association.
However, the Atlas 2017 project does have a number of limitations. In the first place, having collected the data through a patient survey online, this could bias the study results since it is possible that patients with higher disease severity tend to respond more frequently to surveys. Being self-reported patient information, there may be some difficulty ensuring proper SpA diagnosis and accurately establishing the type of axSpA experienced.12,13 On the other hand, it should be noted that the survey did not collect direct non-medical costs related to the transportation of patients to attend consultations, tests, emergencies, and other health services. Likewise, it did not include information about professional and informal care received by patients, nor about adaptations made to housing as a result of physical limitations. Previous studies have shown that patients with advanced AS can incur significant out-of-pocket costs to cover informal care and accommodation.28 Therefore, it was necessary to estimate the cost related to informal care and housing adaptations, taking data from the literature.
The Atlas 2017 has achieved great impact at national level, being published by the Institute Max Weber.29 Likewise, a multitude of informative support content has been generated, including the specific web of the Atlas 2017 (www.espondiloartritisaxial.org/atlas/), infographics, and a documentary aimed at enhancing scientific and social understanding. Proof can be seen in the high number of downloads relating to this document to date. On the other hand, the Atlas data are being presented and discussed with the regional health authorities of Spain (Autonomous Communities) to put the axSpA in the health agendas in order to plan and implement solutions at National Health Service level to improve patient's health.
The Atlas 2017 has been considered a benchmark study. As a result, its methodology is being implemented in 12 other European countries (Austria, Belgium, France, Germany, Italy, Netherlands, Norway, Russia, Slovenia, Sweden, Switzerland and United Kingdom) under a joint project called EMAS (The European Map of Axial Spondyloarthritis). EMAS is promoted by the Ankylosing Spondylitis International Federation (ASIF), CEADE, and the University of Seville. In this way, it is intended to assess the existing inequalities and disparities between countries and establish recommendations aimed at improving patient care in Europe.
ConclusionsThe Atlas 2017 aims to better understand the current reality of people suffering from axSpA from an integrative perspective based on scientific evidence, expert knowledge, and patient opinion. The patient survey data shows still a long diagnostic delay, high disease activity, psychological distress, while an important proportion could be undertreated.
Conflict of interestThe authors declare that they have no conflicts of interest.
Funding: We would like to thank all the patients who volunteered participated in the survey promoted by the Spanish Coordinator of Associations of Spondyloarthritis (CEADE).
This work was supported by Novartis Farmacéutica S.A.