To assess the efficacy and side effects of methotrexate and leflunomide in patients with rheumatoid arthritis (RA) as the first disease-modifying antirheumatic drug (DMARD).
MethodsWe performed a systematic review and meta-analysis of clinical studies that included patients who took methotrexate, leflunomide, placebo or another DMARD for RA treatment. A systematic review yielded 1971 articles from databases; once completely reviewed, 73 trials that completed inclusion criteria were selected. In structured workshops for discussion and assessment of each article, 6 could be meta-analyzed for the primary and secondary outcomes: achievement of American College of Rheumatology (ACR) 20 and its core set components; and change of serum C-reactive protein (CRP) levels, Health Assessment Questionnaire Disability Index (HAQ-Di), liver enzyme aspartate transaminase/alanine transaminase ratio, new gastrointestinal (GI) side effects and infections.
ResultsA total of 1984 patients were included: 986 took leflunomide and 998 methotrexate. The probability of achieving ACR 20 had an odds ratio (OR) of 0.88 (95% confidence interval [CI] 0.74, 1.06) with a trend toward favoring methotrexate; reduction of the swollen joint count was greater for methotrexate: mean difference=0.82 (95%CI 0.24, 1.39); tender joint count, physician global assessment, HAQ-Di, and serum CRP levels revealed no significant difference between groups. Increased liver enzymes were more frequent in the leflunomide group, OR=0.38 (95%CI 0.27, 0.53), and new GI complaints were more common with methotrexate (OR=1.44; 95%CI 1.17, 1.79). There was no difference in the incidence of non-severe infections.
ConclusionLeflunomide used as the first DMARD in RA seemed to be as efficacious as methotrexate; only the reduction of swollen joint count was more marked for methotrexate. Leflunomide was linked to a greater increase in liver enzymes, but there were fewer GI complaints.
Evaluar la eficacia y los efectos secundarios del metotrexato o la leflunomida en pacientes con AR como primer fármaco modificador de la enfermedad (FAME).
MétodosSe realizó una revisión sistemática y metaanálisis de estudios clínicos que incluyeron a pacientes que tomaron metotrexato, leflunomida, placebo u otro FAME para el tratamiento de la AR. Después de una revisión sistemática, se encontraron 1.971 artículos, una vez revisados completamente, se seleccionaron 73 ensayos que completaron los criterios de inclusión. En talleres estructurados para el debate y la evaluación de cada artículo, 6 pudieron ser metaanalizados para los resultados primarios y secundarios: logro de ACR 20 y sus componentes básicos, así como el cambio de los niveles séricos de PCR, HAQ-DI, enzimas hepáticas AST/ALT, nuevos efectos secundarios gastrointestinales (GI) e infecciones.
ResultadosSe incluyó a un total de 1.984 pacientes, 986 tomaron leflunomida y 998 metotrexato. La probabilidad de alcanzar ACR 20 reveló una OR 0,88 (IC del 95%: 0,74; 1,06) con una tendencia a favorecer el metotrexato; la reducción del recuento de articulaciones inflamadas fue mayor para metotrexato: diferencia de medias (MD)=0,82 (IC del 95%: 0,24, 1,39); el recuento de articulaciones sensibles, la evaluación global de médicos, el HAQ-DI, y los niveles séricos de PCR no revelaron diferencias entre los grupos. El aumento de las enzimas hepáticas fue más frecuente en el grupo con leflunomida, OR=0,38 (IC del 95%: 0,27, 0,53) y las nuevas quejas GI fueron más frecuentes en el metotrexato, OR=1,44 (IC del 95% 1,17, 1,79). No hubo diferencias en la incidencia de infecciones no graves.
ConclusiónLa leflunomida utilizada como el primer FAME en la AR parece ser tan eficaz como el metotrexato; solo la reducción de las articulaciones inflamadas fue mayor para el metotrexato. La leflunomida está relacionada con una mayor elevación de las enzimas hepáticas, pero presenta menos molestias GI.
RA is a frequent condition worldwide suffered by around 1.5 million people in our country according to recent surveys.1 This disease is related to several adverse outcomes, including a greater mortality, progressive disability, accrual organ damage, severe medication's side effects, and higher, even catastrophic, direct and indirect costs.2,3 All clinical guidelines encourage the use of disease-modifying anti-rheumatic drugs (DMARDs) since the diagnosis, in order to prevent irreversible joint damage and minimize symptoms of disease. There is evidence that, the earlier use of one DMARD, the higher probability of remission and low progression of disease4; for that reason, such guidelines encourage the use of DMARDs in very early stages of RA, during the first weeks of clinically relevant synovitis.5–7
Since decades ago, methotrexate has been considered the “anchor” drug for RA treatment,8 which means it can be used alone or in combination with both, synthetic and biologic DMARDs, in any stage of RA. This drug exerts a known efficacy of around 70%, 60% and 35% for achievement of ACR-20, -50 and -70, respectively, in patients naïve to DMARDs,9 and also has the same range of efficacy reported in several comparisons with other DMARDs.10 However, methotrexate is frequently related with poor tolerance; 60% of RA patients experienced at least one side effect, with persistent appearance of complaints along all the time that this drug is taken. Currently, folic acid is used to minimize methotrexate side effects; although nausea, vomiting, rise of liver enzymes, and other gastrointestinal discomforts remain in the range of 20–65%, as well as other ceaseless complaints such as alopecia, headache, mouth ulcers and sores. Discontinuation of the drug because of side effects ranges from 10 to 40% in different series, although it is less frequent than observed with other synthetic DMARDs, being as high as 52% for sulfasalazine, 55% for D-penicillamine, 64% for parenteral gold salts, and 14% for antimalarial drugs.11–14
Leflunomide, an isoxazole derivative developed for the treatment of RA, is now broadly used alone or in combination.15 Once administered, it is converted to an active metabolite, teriflunomide (A77 1726), that exerts a potent inhibition of the lymphocyte enzyme dihydroorotate dehydrogenase, within the pyrimidine biosynthetic pathway, resulting in decrease of T-cell proliferation, as well as other changes in the immune response.16 Leflunomide has shown in several clinical trials efficacy comparable to methotrexate for control of signs and symptoms of RA, and has been also related with slowing of joint damage progression.17 On the other hand, information regarding the tolerance and safety of this drug has changed as long as it is used in more patients; for instance, rise of liver enzymes has been found similar to methotrexate, and there are reports of pregnancies with good outcomes in patients who incidentally use of leflunomide,18,19 two previously recognized major adverse effects. Nevertheless, the issue of interstitial lung disease in Asian patients with pre-existing pulmonary lesions remains important for that population.
In less affluent communities, with some barriers for the use of biologic agents, leflunomide is recommended as the same level of methotrexate as initial drug, as monotherapy or in combination; and current evidence suggests that could have some advantages over methotrexate.7 We decided to perform a systematic review and meta-analysis comparing efficacy and safety of methotrexate and leflunomide in RA patients to address the previously suggested superior attributes of leflunomide.
MethodsStudy eligibilityInclusion criteria for studies were as follows: clinical trials, reports of clinical trials, previous systematic reviews, or meta-analysis in which at least two of any of these treatment branches were assessed, methotrexate, leflunomide, other DMARD, or placebo. Patients should be subjects of any gender, 18 years-old of age at the moment of inclusion, with diagnosis of RA according to ACR 1987 classification criteria, or the 2010 ACR/EULAR classification criteria. Once examined all abstracts in search of inclusion criteria, articles proposed to be analyzed were read in extenso by all authors; then in a structured workshop all discrepancies were analyzed and the final acceptance occurred if two authors agree about suitability of the article for meta-analysis, mainly if study design were correct, drug interventions, patients characteristics, and measures of efficacy and safety; therefore, assessment of article quality was performed. Articles were identified by the first author surname and the year of publication; funding and other economic support for the trial performance, and authors competing interest declarations were also registered.
Data sourceSystematic literature searches of the aforementioned clinical trials were searched in The Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library, MEDLINE via PubMed, and Embase databases since January 1st, 1985 to April 30th, 2017. Abstracts of the American College of Rheumatology since 1990, and the European League Against Rheumatology since 2000 annual meetings up to 2016 were also searched. Only studies written in English, Spanish or Portuguese were included (search strategy is depicted in Appendix A).
Study selectionOnce searched in literature sources for clinical trials, abstracts of all manuscripts, and other papers found were reviewed. Articles included for the authors revision workshop should contain a randomized comparison of at least two treatments of one active drug versus placebo or another active drug; studies in which one branch had one biologic agent, DMARD combinations, biologic and synthetic DMARD combinations, or other pharmacological or non-pharmacological interventions were excluded. On the other hand, patients must be naïve to any kind of DMARD, had at least 6 months of disease duration, and presented with active disease at the time of inclusion to the study. Furthermore, clinical trials should have explicit data on doses, schedule, administration path, and duration of treatment with leflunomide or methotrexate; measurements of efficacy with the ACR improvement criteria or the disease activity score of 28 joint count (DAS-28) index; swollen and tender joint counts must also be clearly detailed, and the change of C-reactive protein (CRP) serum levels along the follow-up. Articles must also contain a description of side effects; at least rise of AST/ALT liver enzymes, frequency, characteristics and severity of infectious episodes, and of gastrointestinal complaints. Changes in disease functional capacity measured by the HAQ-Di might be included.
Follow-upPatients were included in the meta-analysis when they completed the controlled phase of study protocol of at least 52 weeks. They should have at least one basal measure of efficacy, and other at least 12 months apart. If the trial had a greater follow-up, only the assessment at one year was taken into account.
OutcomesEfficacy was measured by the change of ACR improvement criteria core set, with the proportion of patients achieved ACR 20, which is defined as: 20% improvement in tender and swollen joint counts, and 20% improvement in 3 of the 5 remaining ACR-core set measures, i.e.: patient and physician global assessments, pain, disability, and an acute phase reactant,20 or the attainment of the DAS-28 in the category of low disease activity or remission, which includes the swollen and tender joint counts, patient global assessment scale, and CRP serum levels. Because the most important variables of both indices was joint counts which are, in experts’ perception, the core set measures for improvement of RA,21 these counts were analyzed separately as main outcome. Secondary outcomes were those related to safety; proportion of patients with rise in AST/ALT serum levels, those who developed an infection registered during study, new gastrointestinal complaints, but also PCR serum levels, and HAQ-Di score changes. Withdrawal of treatment, and dropouts were also registered.
Data collection process and quality assessmentAll data were extracted by one of the authors (RA-L), with a review of data sheets by other (HFE-O). Data were codified in an Excel sheets. Two authors scored all papers separately, with the Jadad scale. Moreover, bias risk of the trials was analyzed with unmasked articles for reviewers in accordance with the statements used in The Cochrane Handbook for Systematic Reviews of Interventions.22 Methods for randomized assignment for each trial was not clearly described; hence, there are no available that issue for analysis in this review. None of the final trials included were supported by pharmaceutical industry, and we believe that the risk of bias was the same for all included articles.
AnalysisTreatment group comparisons were made between leflunomide and methotrexate. For measurements of treatment effects, and other analysis we used RevMan Analyses V5 software. Discrete data were analyzed calculating the odds ratios and their 95% confidence intervals. Continuous data were analyzed comparing their mean differences with their 95% confidence intervals between intervention groups. Forest graphics and p values were obtained with fixed effect analysis, heterogeneity effect among clinical trials were measured with X2 and I2 statistics. Main outcomes are reported accordingly they were reported in the original studies, either per-protocol o for intention-to-treat analysis.
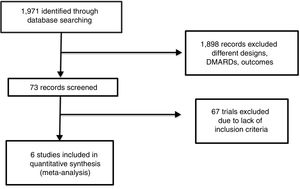
ResultsAccording with the search strategy formerly described, we found a total of 1971 articles in the consulted databases. We selected 73 that fit established selection criteria for inclusion and could be potentially included for meta-analysis; 1898 papers were rejected, mainly because design issues, shorter length of follow up, other written language different of previously established, phase I or II trials, or other observational or experimental designs. After the structured workshop and the whole review of all full-text manuscripts, sixty seven were rejected for the final analysis, mainly because of: lack of design information or data location, outcome measures were incompatible with this review aim, case-definition used did not fit, main objective of original trial was not efficacy or safety assessment. Other reasons for rejection were the use of synthetic DMARDs combination, or the use of biologic agents alone, or in combination with synthetic DMARDs, not specification or change of active drug doses or confusing schedule of such a drug. Finally, we found 6 trials suitable for systematic review and meta-analysis (Fig. 1).
Four of the analyzed trials had the comparison of two drugs: methotrexate and leflunomide (Jaimes-Hernández,23 Ishaq,24 Emery,25 and Cohen26), the remaining two studies had three branches for comparison: methotrexate, leflunomide and placebo (Strand27 and Moreland28) number of patients included in the trial, and its Jadad score are shown in Table 1. It is important to point out that the Emery's trial contribute with almost half of patients in this meta-analysis, Jaimes-Hernández trial was made in Mexican patients, and Ishaq in Pakistani subjects, with the remainder populations of European and North American patients. At the end of the 80s and early 90s a group of investigators reported several trials with leflunomide as a part of its clinical development program, called European Leflunomide Study Group, and the Leflunomide Rheumatoid Arthritis Investigators Group, and despite that some of their trials met our review inclusion criteria, we found that patient population, follow-up, and intervention were the same for those trials,29–32 and for that reason, only Strand's trial was included.
Main characteristics of trials included for meta-analysis.
| Trials included | ||||||
|---|---|---|---|---|---|---|
| Strand27 | Jaimes-Hernandez23 | Ishaq24 | Moreland28 | Cohen26 | Emery25 | |
| Patients included | 482 | 85 | 240 | 183 | 199 | 984 |
| Drugs compared | LEF vs MTX vs Placebo | LEF vs MTX | LEF vs MTX | LEF vs MTX vs Placebo | LEF vs MTX | LEF vs MTX |
| Outcomes measured | Efficacy and side effects | Efficacy and side effects | Efficacy and side effects | Efficacy | Efficacy | Efficacy and side effects |
| Duration | 52wks | 52wks | 52wks | 104wks | 52–104wks | 52–104wks |
| Intention-to-treat | Yes | No | Yes | Yes | Yes | Yes |
| Jadad score | 4 | 3 | 3 | 3 | 4 | 4 |
Abbreviations: LEF, leflunomide; MTX, methotrexate.
A total of 1984 patients were included in these 6 trials, 986 received leflunomide in daily dose of 20mg, after a 100mg daily for three days as load dose, and 998 had methotrexate in doses ranging from 7.5 to 20mg weekly; typically, in this later group drug dose was escalated during the follow-up to reach better clinical response. It is important to highlight that although one of the studies (Jaimes-Hernández) employed a weekly fixed 100mg dose of leflunomide compared to 20mg daily dose in the rest of the trials, the authors describe that there are no differences using the fixed dose in previous studies.
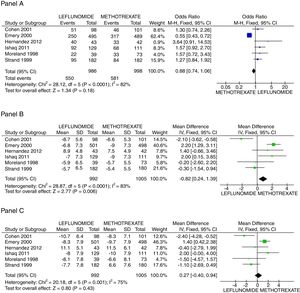
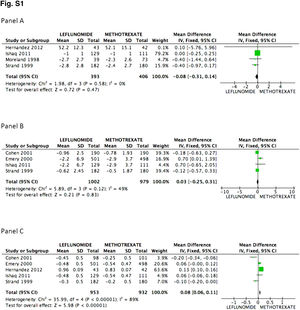
There was no difference in the probability to achieve ACR 20 at 52 weeks between patients receiving leflunomide or methotrexate, odds ratio (OR) 0.88 (95% confidence interval [95%CI 0.74, 1.06; p=NS; I2=82%). On the other hand, the mean difference (MD) between groups in the reduction of the swollen joint count at the end of follow-up was greater for the methotrexate group, MD=0.82 (95%CI 0.24, 1.39; p=0.006; I2=83%); meanwhile difference in the tender joint count between groups did not reach significance, MD=0.27 (95%CI −0.40, 0.94; p=NS; I2=75%). In Fig. 2, panels A, B, and C the forest graphics for these outcomes are shown. As the secondary efficacy outcomes we measured PCR serum levels changes, that could be analyzed only in the Cohen,26 Emery,25 Ishaq,24 and Strand27 trials, and did not depict significant difference between groups with a MD=0.03 (95%CI −0.25, 0.31; p=NS; I2=49%), the same occurred in the comparison of the physician global assessment, MD=−0.08 (95%CI −0.31, 0.14; p=NS; I2=0%) (supplementary material). Finally, regarding assessment of the mean change of the HAQ-Di index, which could be measured in all trials but Moreland, difference favor methotrexate, MD=0.08 (95%CI 0.06, 0.11; p<0.001; I2=89%).
Assessment of efficacy outcomes. Comparison of methotrexate and leflunomide groups are made in these forest plots. Panel A shows the odds to achieve ACR 20, Panel B the mean difference of reduction in swollen joint counts, and Panel C the mean difference in the reduction of tender joints count.
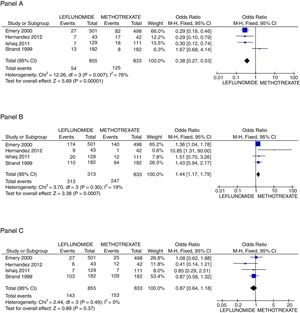
In regard to safety issues, only Emery,25 Ishaq,24 Jaimes-Hernández,23 and Strand27 trials could be analyzed. Comparisons of AST/ALT liver enzymes raise incidence, non-severe infections, and new gastrointestinal complaints were made. It is important to call attention to the absence of deaths in the follow-up of all studies. Rise of liver enzymes was more frequent in the leflunomide group, OR=0.38 (95%CI 0.27, 0.53; p<0.001; I2=76%); whilst the incidence of new GI complaints was greater for the methotrexate group with OR=1.44 (95%CI 1.17, 1.79; p<0.001; I2=19%), and the frequency of non-severe infections during the trial length was not different between groups OR=0.87 (95%CI 0.64, 1.18; p=NS; I2=0%) (see Fig. 3). Finally, regarding patients who withdrew any drug because of adverse effects, only two trials25,26 reported explicitly that patients on leflunomide experienced more often diarrhea (2.0% vs 0.8%), and otherwise, the rate of withdrawn was similar between both groups.
Principal safety outcomes evaluated. Panel A shows the forest plot assessing the odds for raising in AST/ALT liver enzymes comparing patients with leflunomide or methotrexate, Panel B the odds for appearance of new gastrointestinal symptoms, and Panel C the probability to present a non-severe infection.
Leflunomide is the last synthetic DMARD developed and investigated for the treatment of RA, which remains as its unique indication in the western countries, since its approval by the FDA in 1998. It was extensively proved in Europe and the US during the late 80s and 90s with good results,27–32 and nowadays it is broadly used worldwide. Some national guidelines recommended its use in early disease; although clinicians still prefer to start therapy with methotrexate. In the ACR 2012 update recommendations for the use of DMARDs in RA,5 no specific preference of one drug over other in the initial monotherapy approach is suggested; furthermore, in patients with poor prognostic factors, no combination is preferably indicated, but frequently those based in methotrexate are initially used. On the other hand, EULAR task force,33 as well as other national official statements,7 recommends that every recently diagnosed patient should be assigned to methotrexate treatment and, if contraindicated, other synthetic DMARD could be used. However, although the analyzed evidence for these statements is categorized like 1A in such guidelines, trials referred had some methodology issues, and could not be used to assess face-to-face leflunomide versus methotrexate comparison; even more, no systematic review or meta-analysis was used for this purpose.
Included trials in this meta-analysis, in which leflunomide and methotrexate are compared, were conducted in the 80s and 90s, and there are no available more recent comparisons; hence, assessment of efficacy was performed only with the ACR improvement core set of disease activity measures, as well as its components.20 This index has been gradually abandoned because its original purpose was to measure differences in clinical response of patients receiving active treatment or placebo in clinical trials, but cannot describe patient's disease activity status at specific point of time, or changes along follow-up in a cohort,34 resulting in lack of sensitivity to change and less responsiveness. Moreover, its scoring system as a categorical scale is at least arbitrary, limiting recognition of intermediate points of improvement, i.e.: between 50% and 20% or 70% and 50%, which determine its unfeasibility in follow-up of individual patients35; however, ACR core set of disease activity measures, and achievement of ACR 20 has been demonstrated the greatest validity to differentiate improvement of one group to another in clinical trials.36 DAS 28 index is widely used now in the assessment of treatment effectiveness; its reduction is considered as the main objective in the treat-to-target strategy, although it was not measured in these trials. Some of its components, also included in the ACR core set were analyzed separately, as the swollen and tender joint counts, physician global assessment (patient global assessment was not uniformly mentioned), and PCR serum levels. In this regard, measured core components, as well as the ACR core set index, but swollen joint reduction, did not disclose difference between methotrexate and leflunomide groups as is shown in the forest graphics.
Long-term survival of one DMARD depends on its efficacy and safety; approaches to evaluate the persistency and compliance of different synthetic DMARDs have been carried out elsewhere. Grijalva et al. evaluates persistence and adherence of newly prescribed DMARDs in a large cohort in Tennessee,37 and considering methotrexate as the drug of reference, they found that among biologic and synthetic DMARDs, leflunomide had greater adherence, according to its medical possession ratio, than other compared therapies, such as sulfasalazine and infliximab which have shown lower adherence rate. In the safety analysis in this study, we found that leflunomide patients disclose greater odds to have raise in liver enzymes, although the total of drug withdrawal were equal for both, leflunomide and methotrexate groups, supporting the notion that not all increases in liver enzymes must be followed by its removal. On the other hand, we found a greater probability of new gastrointestinal complaints in the methotrexate group, a finding fitting with information related to low tolerance of this drug, mainly because of such complaints. Taking into account the requirement of drug withdrawal, which did not differ between treatment groups, side effects were of the same importance for methotrexate, sulfasalazine, or leflunomide as it was reported by Osiri38 in a previous systematic review and meta-analysis. In this regard, there is only one previous meta-analysis comparing efficacy of leflunomide, published as an article,38 and in deep detail in the Cochrane database library39; although for the assessment of methotrexate and leflunomide efficacy only a few trials are included, but in general terms, their results are consistent with ours.
One of the main limitations of the present study is that the authors focused only on two different DMARDs instead of having a broader (at least three drugs) comparison. Although is important for rheumatologists to have a full evaluation among the different options of synthetic DMARDs, our aim was to analyze whether methotrexate could be stated as a first line treatment option according to the EULAR recommendations. Regarding to this, only methotrexate and leflunomide, both as a monotherapy, have shown to slow radiographic progression31,40 compared to other synthetic drugs. Another important limitation is that our results did not consider economic aspects of the studied drugs. It is known that leflunomide might be more expensive than methotrexate even in generic formulation, but such evaluation was not performed as it is beyond the scope of this trial.
In conclusion, this meta-analysis demonstrates that methotrexate and leflunomide exerts the same efficacy in patients with RA naïve to other DMARDs, and despite their different profile of tolerance or side effects, rate of maintenance remains equal between trials; therefore, both drugs can be used as the first choice in patients with active RA. Longer comparisons to assess other relevant outcomes, such as disability, structural damage, or preservation of job, as well as economic evaluations of these drugs are warranted.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare that they have no conflicts of interest.
[arthritis OR rheumatoid OR rheumatoid arthritis OR rheumatic OR RA OR rheumatic disease OR autoimmune rheumatic disease OR rheumatism OR mild rheumatoid arthritis OR moderate rheumatoid arthritis OR severe rheumatoid arthritis]AND[disease modifying antirheumatic drugs OR DMARD OR DMARDS OR methotrexate and lefunomide OR methotrexate vs leflunomide OR leflunomide OR methotrexate OR lfl OR MTX OR antirheumatic OR drugs OR antifolate drug OR antimetabolite drug OR pyrimidine synthesis inhibitor]AND[randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR drug OR therapy OR drug therapy OR randomly OR trial OR groups OR humans trial OR clinical trial as topic].