End-stage renal disease (ESRD) due to lupus nephritis (LN) occurs in 10%–30% of patients. Initially systemic lupus erythematosus (SLE) was a contraindication for kidney transplantation (KT). Today, long-term graft survival remains controversial. Our objective was to compare the survival after KT in patients with SLE or other causes of ESRD.
MethodsAll SLE patients who had undergone KT in a retrospective cohort were included. Renal graft survival was compared with that of 50 controls, matched for age, sex, and year of transplantation. Survival was evaluated by the Kaplan–Meier test and the Cox proportional hazards model.
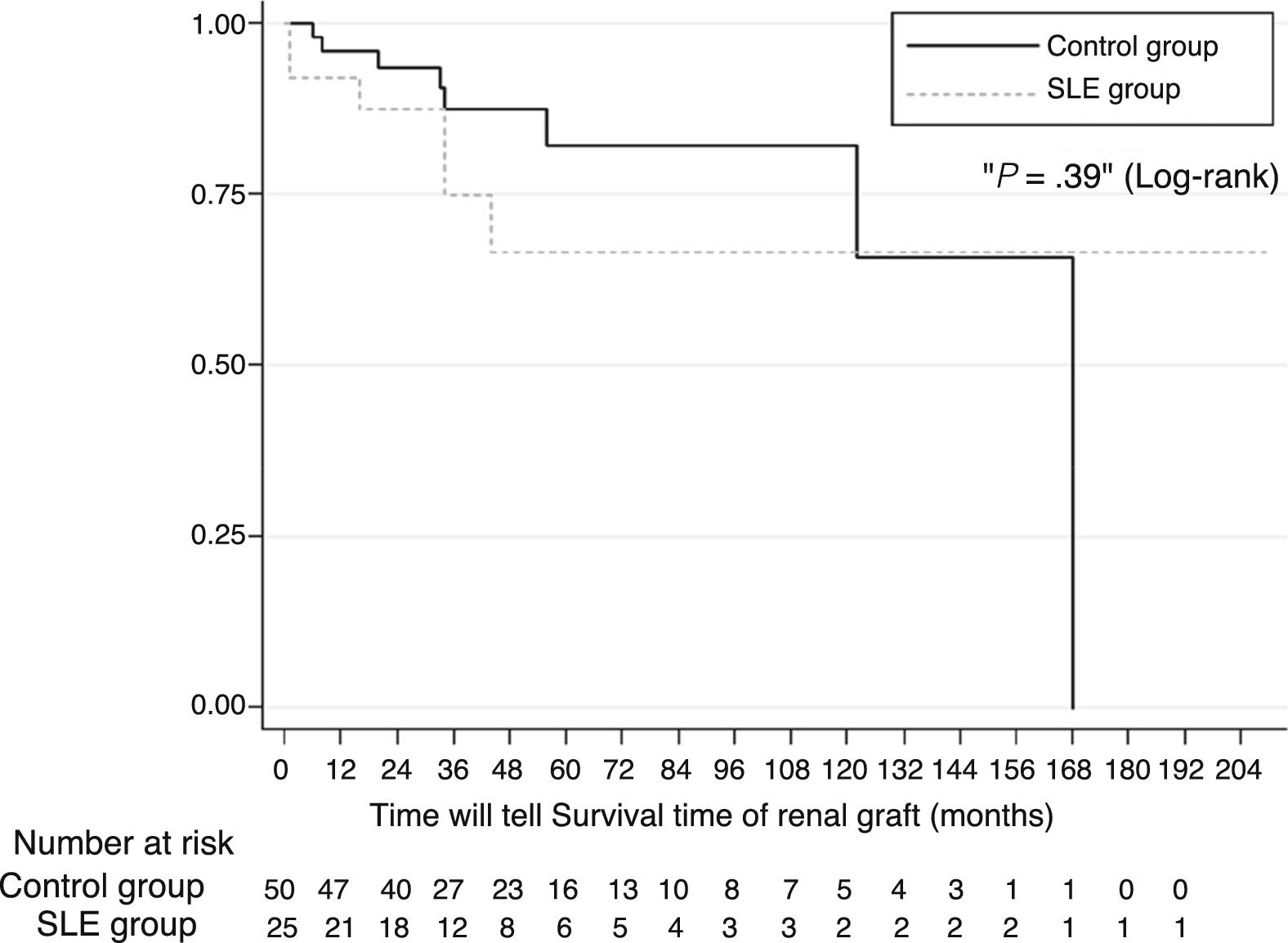
ResultsTwenty-five subjects with SLE were included. The estimated 1-year, 2- and 5-year survival rates for patients with SLE were 92%, 66% and 66%. Renal graft survival did not differ between patients with SLE and other causes of ESRD (P=.39). The multivariate analysis showed no significant difference in graft survival between the two groups (hazard ratio, HR=1.95, 95% confidence interval [CI] 0.57–6.61, P=.28). The recurrence rate of LN was 8% and was not associated with graft loss. Acute rejection was the only variable associated with graft loss in patients with SLE (HR=16.5, 95% CI 1.94–140.1, P=.01).
ConclusionsRenal graft survival in SLE patients did not differ from that reported for other causes of ESRD.
La enfermedad renal terminal (ERT) por nefritis lúpica (NL) se presenta en el 10-30% de los pacientes. Inicialmente, el lupus eritematoso sistémico (LES) fue una contraindicación para el trasplante renal (TR). En la actualidad, la supervivencia del injerto a largo plazo sigue siendo motivo de controversia. El objetivo del estudio fue comparar la supervivencia del TR en los sujetos con LES con otras causas de ERT.
MétodosSe incluyó a todos los pacientes con TR en sujetos con LES, de una cohorte retrospectiva en 2 centros de trasplante. Se realizó un grupo de comparación con otras etiologías de ERT en una relación 2:1 emparejados por edad, sexo y año del trasplante. La supervivencia se evaluó por el método de Kaplan-Meier y por el modelo de riesgos proporcionales de Cox.
ResultadosSe incluyó a 25 sujetos con LES. La probabilidad de supervivencia en los sujetos con LES al año, 5 y 10 años fue del 92, el 66 y el 66%, respectivamente, la cual no difirió del grupo de comparación (p=0,39). En el análisis multivariante no existió una diferencia significativa en la supervivencia del injerto entre los 2 grupos (hazard ratio=1.95, IC del 95%, 0,57-6,61; p=0,28). La recurrencia de la NL fue del 8% y no se relacionó con la pérdida del injerto. El rechazo agudo fue la única variable asociada con la pérdida del injerto en los sujetos con LES (HR 16,5, IC del 95%, 1,94-140,1, p=0,01).
ConclusionesEl riesgo de pérdida del injerto renal en los sujetos con LES fue similar al de los sujetos con otras causas de ERT.
It has been estimated that during the course of their disease 60% of patients with systemic lupus erythmatosus (SLE) will present with some level of nephritis.1 Despite the fact that in the last few decades treatment and prognosis of lupus nephritis (LN), has improved, between approximately 10% and 30% of patients develop end-stage renal disease (ESRD),1–3 which requires kidney replacement therapy.
Initially SLE was considered a contraindication for kidney transplant (KT) due to the high probability of recurrence of LN (RLN) and to high morbidity. This opinion changed in 1975 when the Advisory Committee to the Renal Transplantation Registry of the American College of Surgeons (ACS) and the National Institute of Health (NIH) concluded that KT in lupus was successful and with similar outcomes to those obtained with the more common causes of ESRD.4 From then onwards, KT began to be considered a reasonable treatment option.
It is believed that ethnic/racial differences are a determining factor in the outcome of NL. Women with Hispanic and Afro-American origins are at higher risk of progression to ESRD when compared with Caucasians.5 Low socio-economic status, the Afro-American race and people of Hispanic/Latin American origin at greater risk of kidney graft loss.6,7 The Afro-Americans have a lower kidney graft survival rate compared with Hispanics, particularly due to a higher rate of graft rejection from cadaveric donor.7 In people from Latin American countries, observational studies which have compared survival of the graft with other causes of ESRD report a similar graft survival to that of comparison groups,8–10 and in the study in Brazil which included mainly Caucasian individuals (91%) they reported better survival of the graft in the group with SLE.11
In transplant centres the frequency of KT in patients with SLE varies between .01% and 5.5% of all KT.9,12–16 Survival of the graft is a subject of great interest due to the long-term prognosis of the graft and the rate of RLN continuing to be a controversial point.4,17,18 The aim of our study was to compare survival of the kidney graft in patients transplanted by LN with other causes of ESRD.
MethodsA retrospective cohort study. Patients who met with the SLE classification from the Americano College of Rheumatology19 were included and who had undergone a KT for LN in 2 third-tier hospitals, during the period between 1st January 1991 and 31st May 2010 in the Centro Médico Nacional (CMN) Siglo XXI (CMN SXXI), and during the period from January 2003 to December 2014 in the CMN “La Raza”. Graft loss was defined by the need for dialysis or creatinine >5mg/dl in 2 tests. Patients with diabetes or who had had transplants due to another autoimmune diseases were excluded. To assess if survival difference from other causes of lo ESRD, 2 controls were selected for each patient with SLE, chosen randomly from the KT patient database, paired by age, gender and date of transplant.
Statistical AnalysisThe results obtained are presented in the form of the absolute number of cases and their percentage (n [%]) for categorical variables, and the mean with its standard deviation (mean±SD) for continuous variables with normal distribution and the median with its interquartile range (IQR) for continuous variables which do not follow this distribution. For comparison of categorical variables in accordance with the study group, the Pearson Chi-square test was used or the Fisher exact test. Graft survival was analysed with the Kaplan–Meier method and the survival curves were compared with the log-rank test. To analyse the variables which impacted the graft loss rate depending on time we used Cox's proportional risk model which controlled the covariables believed to be probable factors of confusion: disease status (SLE compared with control), type of transplant, age of transplant, dialysis time, post-transplant thrombosis, acute rejection, chronic graft nephropathy and immunosuppressant treatment used. The validity of each model was assessed with the Shoenfield residuals test method. A P value of <.05 was considered statistically significant. The statistical programme Stata-13 (StataCorp. 2013. Stata-13 Statistical Software: Release 13. College Station, TX: StataCorp LP) was used for statistical analysis.
ResultsCharacteristics of the Population Under StudyDuring the study period 1749 kidney transplants were made in the CMN SXXI, 13 were patients with SLE (.8%) and 4 cases were eliminated due to incomplete information. In the “La Raza” CMN 16 kidney transplants were performed in subjects with SLE. The final sample comprised 25 patients with SLE, 5 of whom were SLE of young-age onset, the first transplant was performed in 1993 and the last in 2014.
Median age at diagnosis of SLE was 20.5 years (IQR 8 years, minimum 10 and maximum 50). Most of the patients were women (19 [76%]), median age at time of transplant was 27 years (IQR 13; minimum 20, maximum 52). No significant differences (Table 1) were observed when comparing the general characteristics between the groups studied. Median therapy duration time for replacement of kidney function was 24 months (IQR 36; minimum 0, maximum 72) in the group with SLE and 26.5 months (IQR 27; minimum 0, maximum 95) in the comparison group; this difference was not statistically significant (P=.67). The most frequent type of kidney replacement prior to transplant in both groups was peritoneal dialysis.
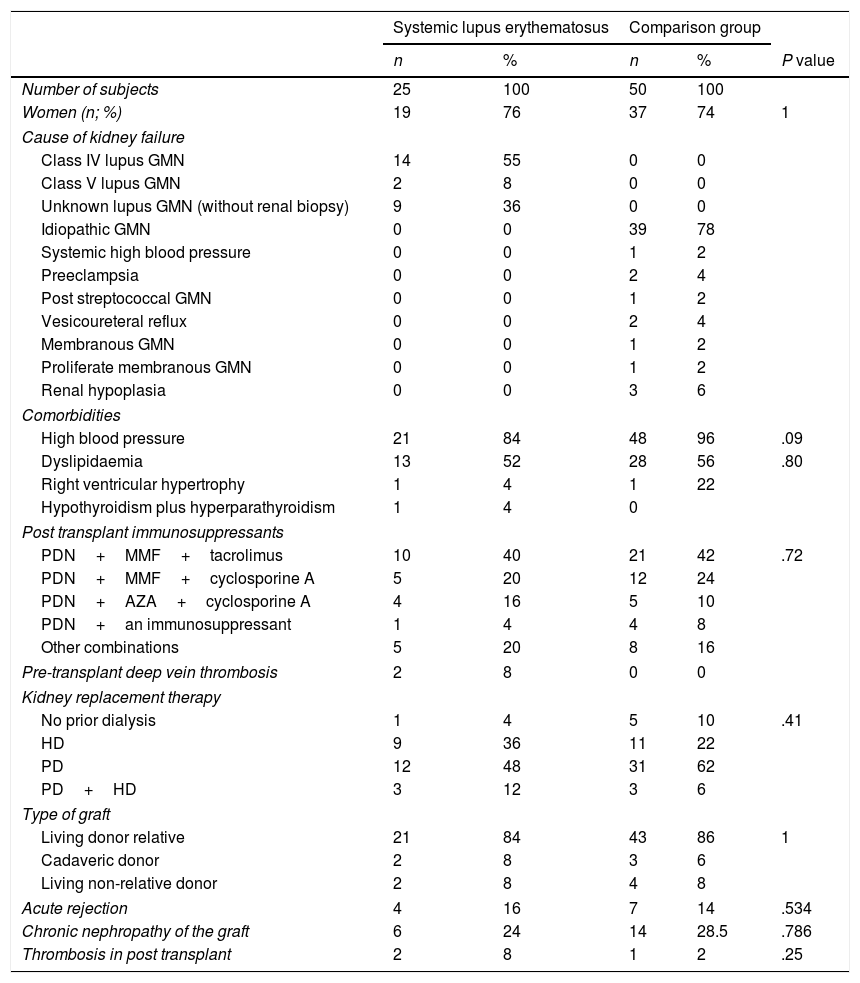
Demographic Traits of Patients With Kidney Transplant Due to Lupic Nephritis and Other Causes of Terminal Kidney Disease.
| Systemic lupus erythematosus | Comparison group | ||||
|---|---|---|---|---|---|
| n | % | n | % | P value | |
| Number of subjects | 25 | 100 | 50 | 100 | |
| Women (n; %) | 19 | 76 | 37 | 74 | 1 |
| Cause of kidney failure | |||||
| Class IV lupus GMN | 14 | 55 | 0 | 0 | |
| Class V lupus GMN | 2 | 8 | 0 | 0 | |
| Unknown lupus GMN (without renal biopsy) | 9 | 36 | 0 | 0 | |
| Idiopathic GMN | 0 | 0 | 39 | 78 | |
| Systemic high blood pressure | 0 | 0 | 1 | 2 | |
| Preeclampsia | 0 | 0 | 2 | 4 | |
| Post streptococcal GMN | 0 | 0 | 1 | 2 | |
| Vesicoureteral reflux | 0 | 0 | 2 | 4 | |
| Membranous GMN | 0 | 0 | 1 | 2 | |
| Proliferate membranous GMN | 0 | 0 | 1 | 2 | |
| Renal hypoplasia | 0 | 0 | 3 | 6 | |
| Comorbidities | |||||
| High blood pressure | 21 | 84 | 48 | 96 | .09 |
| Dyslipidaemia | 13 | 52 | 28 | 56 | .80 |
| Right ventricular hypertrophy | 1 | 4 | 1 | 22 | |
| Hypothyroidism plus hyperparathyroidism | 1 | 4 | 0 | ||
| Post transplant immunosuppressants | |||||
| PDN+MMF+tacrolimus | 10 | 40 | 21 | 42 | .72 |
| PDN+MMF+cyclosporine A | 5 | 20 | 12 | 24 | |
| PDN+AZA+cyclosporine A | 4 | 16 | 5 | 10 | |
| PDN+an immunosuppressant | 1 | 4 | 4 | 8 | |
| Other combinations | 5 | 20 | 8 | 16 | |
| Pre-transplant deep vein thrombosis | 2 | 8 | 0 | 0 | |
| Kidney replacement therapy | |||||
| No prior dialysis | 1 | 4 | 5 | 10 | .41 |
| HD | 9 | 36 | 11 | 22 | |
| PD | 12 | 48 | 31 | 62 | |
| PD+HD | 3 | 12 | 3 | 6 | |
| Type of graft | |||||
| Living donor relative | 21 | 84 | 43 | 86 | 1 |
| Cadaveric donor | 2 | 8 | 3 | 6 | |
| Living non-relative donor | 2 | 8 | 4 | 8 | |
| Acute rejection | 4 | 16 | 7 | 14 | .534 |
| Chronic nephropathy of the graft | 6 | 24 | 14 | 28.5 | .786 |
| Thrombosis in post transplant | 2 | 8 | 1 | 2 | .25 |
AZA: azathioprine; Pd: peritoneal dialysis; HD: haemodialysis; MMF: mycophenolic acid; PDN: prednisone.
In subjects with SLE graft origin was mainly (84%) from a live donor who was a relative and in no case was SLE activity present during transplant or was there any hepatitis C virus infection. None of the patients had a secondary antiphospholipid syndrome (APS). In 7 of the 25 patients at least one determination of aniphosphlipid antibodies (APA) was performed and all tested negative. Prednisone (92%), mycophenolic acid (76%) and tacrolimus (48%) were the most commonly used immunosuppressants after transplant. In the comparison group there was a higher rate of systemic high blood pressure (96% vs 84%, P=.09). Median post-transplant follow-up time was 34 months (IQR 27) for patients with SLE and 36 months (IQR 48) for patients of the control group.
Kidney Graft OutcomesSurvival probability calculated using the Kaplan–Meier (Fig. 1) method on comparing patients with LN and yearly controls (92% compared with 95%), 2 years (87% compared with 85%), 5 years (66% compared with 87%) and 10 years (66% compared with 65%) was similar. The log-rank test did not show any significant difference in survival over time (P=.39). Survival proportion adjusted to death, estimated at 1, 5, and 10 years was 93%, 72% and 72%, respectively, in the LN group and 97%, 83% and 66%, respectively in the comparison group. In multivariate analysis there was no significant difference in survival of the graft between the 2 groups (hazard ratio [HR]=1.95, 95% CI, .57–6.61; P=.28).
When comparing the group of patients with SLE and the control group no statistically significant differences were observed in graft loss (6 [24%] vs 8 [16%], OR 1.65 [95% CI, .5–5.44], P=.53), the frequency of acute rejection (4 [16%] vs 7 [14%], OR 1.17 [95% CI, .3–4.45], P=.81) and chronic nephropathy of transplant (6 [24%] vs 15 [30%], OR 0.73 [95% CI, .24–2.21], P=.78) and deaths (2 [8%] vs 2 [4%], OR 2.08 [95% CI, .27–15.7], P=.59). The reasons associated with graft loss per group are contained in Table 2. As a protocol of the institution, early or late dysfunction of the graft led to diagnostic biopsy to establish changes in the immunosuppressant regime or detect dysfunction of the graft or other reasons. In the SLE group 12 cases presented and 18 in the control group.
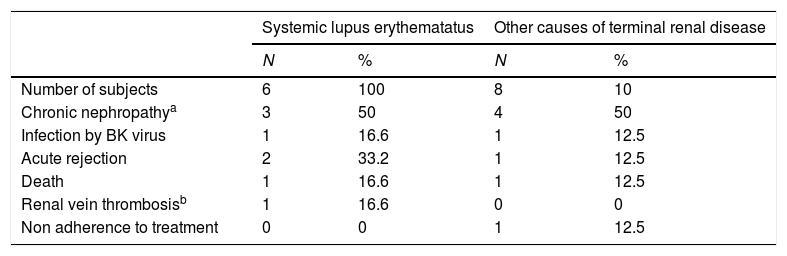
Causes Associated With the Loss of the Renal Graft in the Lupus Nephritis Group and Other Causes of Terminal Renal Disease.
| Systemic lupus erythematatus | Other causes of terminal renal disease | |||
|---|---|---|---|---|
| N | % | N | % | |
| Number of subjects | 6 | 100 | 8 | 10 |
| Chronic nephropathya | 3 | 50 | 4 | 50 |
| Infection by BK virus | 1 | 16.6 | 1 | 12.5 |
| Acute rejection | 2 | 33.2 | 1 | 12.5 |
| Death | 1 | 16.6 | 1 | 12.5 |
| Renal vein thrombosisb | 1 | 16.6 | 0 | 0 |
| Non adherence to treatment | 0 | 0 | 1 | 12.5 |
a One patient presents with chronic nephropathy of the graft plus infection by the BK virus.
b The same patient presented with acute rejection in addition to renal vein thrombosis.
In the cohort analysis of patients with SLE, the year in which the transplant was performed did not have any significant impact on the frequency of graft loss (0 losses in 2 KT between 1990 and 1999, 3 in 10 KT between 2000 and 2009 and 3 out of 7 KT between 2010 and 2015, P=.65). The number of shared haplotypes between the donor and recipient was not associated with graft loss (P=.19) in analysis of the 9 patients where information was present (2 with graft loss and 7 without). Of the 4 patients (16%) with SLE who presented with episodes of acute rejection, one patient presented with acute vascular rejection in addition to venous thrombosis of the graft, one patient presented with acute grade IA rejection and 3 presented with acute grade IIA rejection fro Banff classification. The outcomes of these patients were: one developed chronic nephropathy from the graft, one continued with a functional renal graft and the other presented with RLN. The patient who presented with acute vascular rejection and thrombosis of the renal vein 6 days after transplant was treated with pulsed methylprednisolone and thymoglobulin. It was not possible to prevent graft loss; this patient had a background of deep vein thrombosis prior to transplant without APA and received perioperative anticoagulation.
Biopsies of two patients (8%) reported renal RLN of diffuse type in the graft. One patient presented with proteinuria in nephritic ranges due to class IV LN of the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification, which was treated with an increase in the dose of glucocorticoids and immunosuppressants, obtaining remission of the activity without any loss of graft. The other patient was treated with cyclophosphamode intravenously with remission of the LN, and later developed chronic nephopathy of the graft and finally loss of the graft 16 months after transplant.
In the cohort of patients with SLE in the multivariate model dialysis time (HR .99, 95% CI, .96–1.03, P=.95), graft type (HR 1.38, 95% CI, .42–4.57, P=.59), the presence of young-age onset SLE (HR 2.34, 95% CI, .32–16.88, P=.39), chronic nephropathy of the graft (HR 1.25, 95% CI, .22–6.93, P=.79) and RLN (HR 2.14, 95% CI, .24–18.42, P=.48) did not predict graft loss. The model which best explained graft loss in patients with SLE was comprised by acute rejection variables (HR 16.5, 95% CI, 1.94–140.1, P=.01), use of mycophenolic acid (HR 0.3, 95% CI, .04–2.31, P=.25) and cyclosporine A (HR .22, 95% CI, .02–2.01, P=.18); the last 2 variables have a protective effect but without obtaining any statistical significance.
DiscussionIn our study, overall survival of the graft using Kaplan–Meier curve assessment demonstrated that the survival rate was similar to the comparison group. However, after adjusting for possible factors of confusion, the receptors with SLE demonstrated an increase in the risk of 95% of graft loss compared with the control group, but this difference was not statistically significant, which could be due to the low statistical strength of the study and future research is required with a larger number of subjects to rule out this possibility. The importance of adjustment of possible confusing variables comes from the fact that risk may change when they are included in analysis. In the study by Chelamcharla et al.,20 better survival of the graft was found in the subjects with SLE in the Kaplan–Meier analysis but the multivariate analysis demonstrated a worse outcome. In contrast, Ward21 found in the univariate analysis that there was a risk of graft loss which was considerably higher in the SLE patients but the risk was similar in the multivariate analysis.
Most studies which compare survival between SLE patients with other causes of kidney disease report a similar survival rate between the groups,3,10,22–29 although others report a lower survival of the graft and slightly higher rates of graft rejection in subjects with SLE.13,20,30,31 This discordance may be due both to the population included and to different follow-up times, to heterogeneity in comparison group selection (historic controls, inclusion or not of diabetics, different SLE glomerulonephritis or all of the causes of ESRD without SLE), or the year of publication. Survival in 1975 at 2 years was 66%4; in the nineties 5 and 10 year survival rates were 68%–81.7%, 45.9%–80% and 18.5%, respectively,13 and in the last decade this has increased to 89.5%–100%, 67%–93% and 57%–86%,13,18,24 respectively. Survival at 15 and 20 years is now 62%–73%3,24,28 and 33%–52%,20,24 respectively.
In studies with Latin American patients graft survival is similar to the comparison groups.8,9 In Colombian subjects, reported survival is 92%–96% at one year, 82%–83% at 5 years and 71% at 15 years8,10; for Mexican subjects it is 94% at one year, 74%–79.9% at 5 years and 73% at 10 years32; and in Brazilian subjects it is 93% at one year, 80%–90.9% at 5 years and 68%–85.7% at 10 years.11,17,18 The RLN would vary between 0 and 54% in the studies3,18,33; this wide range may be due to ethnicity and whether the biopsy was performed by protocol or due to medical indication. The clinically significant recurrence in the studies was found to be between 2% and 19%,4,6,22,23,29 and by protocol between 30% and 54%.3,28 In Latin American countries, the RLN according to the country of study was: Mexico 4%–7.4%9,32; Colombia 3%8,10 and Brazil 0%–11%.11,18 Although RLN increases the risk of graft failure, only 2%–7% of graft losses are attributable to this, compared with 43% attributable to rejection.29,31,34 Similarly to our results, acute rejection is a factor significantly associated with graft loss in patients with SLE, although risk of acute rejection is no different from the comparison group risk.8,32 The frequency of acute rejection in patients with SLE varies between studies. Some report a higher rate.28,35 others report a similar frequency to the comparison group3,20,21,30 and, in contrast Yu et al.23 report a lower frequency.
Controversy still exists regarding patients with SLE about what factors are associated with renal graft loss, and the most commonly described are: being a younger recipient. Not receiving induction therapy, having immunosuppressant therapy with 2 drugs, multiple blood transfusions, a higher comorbidity index, higher body weight, donor or recipient of Afro-American race, cadaveric donor, time in dialysis, a lower number of shared haplotypes, tobacco consumption, the presence of acute rejection, lack of treatment adherence, thrombosis of the artery or renal vein and delay in graft function.1,3,13,17,22,24,30,31,34 In contrast, the use of mycophenolic acid, tacrolimus and the absence of APA appear to be related to better survival of renal graft.22 The persistence of serological changes (raised dsDNA and/or hypocomplementemia) during KT is not associated with graft loss.36
As with other aetiologies of ESKD, the main cause of graft loss is chronic nephropathy of the graft (8%–35%).8,23,37,38 In our study, a patient in treatment with cyclosporine after transplant presented with toxicity by cyclosporine, a risk factor of chronic nephropathy of the graft,39 with an improvement in the kidney function after its suspension and no development of chronic nephropathy.
Similarly to that described,9,15,18,32 no association was found between the loss of transplant and the time in dialysis. In clinical practice, the best transplant time is open to controversy. In general, most authors recommend starting dialysis of the transplant and waiting until the SLE is in complete from 6 to 12 months,9,14,23 especially between fast progression to ESKD to verify that no spontaneous recuperation of kidney function had occurred.40 In contrast, other studies have demonstrated a better result in patients with less time in dialysis22,29,41 and without previous dialysis.4,33,39
Because thrombotic events usually result in graft loss, from absence of collateral blood vessels, the presence of APA is a concern due to the risk of thrombosis. In some studies a higher risk of thrombosis has been described in SLE subjects,9,15,27,28,42,43 while in others thrombosis as a cause of graft failure is reported as no different to the control groups.3,20,44 In our study, one patient with SLE with negative APA on 3 occasions and with no obstetric morbidity presented with thrombosis of the renal vein. Diagnosis of APS cannot be ruled out totally for 2 reasons: the possibility of presenting with antibodies aimed against phospholipids or cofactors could exist which are not assessed in clinical practice (prothrombin, phosphatidylethanolamine, annexin v or the vimentin/cardiolipin complex) which could cause the seronegative APS.45 The case has been described of 2 subjects who presented with repeated renal graft loss due to thrombotic complications with negative APA, which were positive prior to the 3 transplant.22 APA may play a role in the early loss of the graft, and it is therefore recommended that their repeated, systematic detection be made during the pre-transplant period.4,18,22
The patient profile with SLE who has undergone KT differs from that of other ESRD causes, and particularly because they are younger, there is a predominance of women and there are fewer comorbidities.3 Despite the previously described controversies, KT may be the best alternative of replacement for kidney function because it offers significantly better survival than peritoneal dialysis or haemodialysis,3,4,46,47 the prognosis at 10 years is comparable with other ESRD aetiologies, it may offer better quality of life in comparison to dialysis, and it is lower in cost in the long term.6
Our results may be interpreted with reserve due to their limitations, which include: a low number of cases, the retrospective design of our analysis and that review of clinical records could not be obtained in all cases of risk factors described. However, we believe it is useful to have the description of the KT outcomes in our population, since our results are consistent with those of previous studies. Notwithstanding, there is a need for future studies where systematic recording of the before-mentioned risk factors should be included to produce a prospective type analysis.
Ethical ConsiderationsThe study was conducted in accordance with the ethical guidelines of research in human beings. It was approved by the local ethical and research committees in both hospital and is registered as: R-2014-3501-4 and R-2011-3601-75.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that for this research no experimentation has been carried out on human beings or animals.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestThe authors declare they have not received any financing and there are no conflicts of interests.
Please cite this article as: Horta-Baas G, Camargo-Coronel A, Miranda-Hernández DG, Gónzalez-Parra LG, Romero-Figueroa MS, Pérez-Cristóbal M. Trasplante renal en lupus eritematoso sistémico: comparación de la supervivencia del injerto con otras causas de enfermedad renal terminal. Reumatol Clin. 2019;15:140–145.