To identify tools designed to evaluate the severity of patients with rheumatoid arthritis (RA) in order to use them in the investigation of prognostic markers in early arthritis.
MethodsWe conducted a systematic review of studies that developed/validated an index for RA disease severity. They were analysed using the COSMIN checklist to assess their methodological quality. In addition, all the variables included were evaluated for their clarity of definition, feasibility and probability of being present in each outcome during the first 2 years of the disease course. To estimate redundancy, variables were grouped by domains.
ResultsAfter reviewing 3519 articles, 3 studies were included. The first study, the PAS, assessed whether current and lifetime treatment with disease-modifying antirheumatic drugs and/or biologics accurately predicted RA severity, as measured by the patient-reported PAS. Treatment variables did not fully distinguish patients in the highest and lowest quartiles of PAS scores. Another severity index, the Claims-Based Index for RA Severity (CIRAS), included the variables age, sex, Felty's syndrome, number of rehabilitation and rheumatology visits, test for inflammatory markers, number of chemistry panels/platelet counts ordered and rheumatoid factor test. The correlation was low (r=0.56) with an index previously validated by the same research group, the RA medical records-based index of severity (RARBIS), with Disease Activity Score-C-reactive protein (DAS28-PCR) (r=0.07) and Multidimensional Health Assessment Questionnaire (MD-HAQ) (r=0.008). Finally, the RARBIS, used to validate the CIRAS, was devised as an RA severity index based on medical records. It includes as domains surgery, radiology, extra-articular manifestations, clinical and laboratory variables, previously chosen by an expert panel. RARBIS had a weak correlation with treatment intensity (r=0.35) and with DAS28 (r=0.41).
ConclusionThere is no index to assess the severity of RA based on the course of the first 2 years of follow-up that is adapted to the current strategy of therapeutic management of this disease. Therefore, we believe it is reasonable to develop a new ad hoc severity index for patients with early arthritis.
Identificar herramientas diseñadas para evaluar la gravedad global de los pacientes con artritis reumatoide (AR) para su uso en la investigación de marcadores pronósticos de artritis precoz.
MétodosRevisión sistemática de estudios cuyo objetivo fuera el desarrollo o validación de índices de gravedad en AR. Se valoró la calidad metodológica mediante la lista de comprobación COSMIN. Además, se evaluó la claridad de definición, viabilidad y probabilidad de estar presente durante los 2 primeros años de evolución.
ResultadosDespués de revisar 3.519 artículos, se identificaron 3 índices de gravedad. La Patient Activity Scale (PAS) valoró si el tratamiento previo o actual predecía la gravedad de la AR, medida mediante el patient-reported PAS. Las variables de tratamiento no permitieron distinguir entre los cuartiles superior e inferior de la PAS. El CIRAS incluye las variables edad, sexo, síndrome de Felty, número de visitas al reumatólogo y al rehabilitador, factor reumatoide (FR), recuento de plaquetas, marcadores inflamatorios y paneles bioquímicos solicitados. Su correlación fue baja (r=0,56), con un índice previamente validado por el mismo grupo investigador, el RARBIS, con el DAS28-PCR (r=0,07) y el Multidimensional Health Assesment Questionnaire (MD-HAQ) (r=0,008). Por último, el RARBIS, utilizado para validar el CIRAS, fue ideado como un índice de gravedad de AR basado en registros médicos. Incluye como dominios cirugía, radiología, manifestaciones extraarticulares, clínica y variables de laboratorio, elegidas previamente por un panel de expertos. Este índice presentó una correlación débil con la intensidad de tratamiento (r=0,35) y con el DAS 28 (r=0,41).
ConclusiónNo existe ningún índice para valorar la gravedad de la AR sobre la base del curso evolutivo de los 2 primeros años de seguimiento y que se adapte a la estrategia terapéutica actual. Por lo tanto, creemos razonable el desarrollo de un nuevo índice de gravedad ad hoc para pacientes con artritis de reciente comienzo.
Recommendations for rheumatoid arthritis (RA) management uphold intensive strategy practices, since scientific evidence has shown that they improve the course of the disease.1–3 These intensive strategies are based on 2 key factors: (a) tight control [TC])4–6 of the inflammatory process and (b) the establishment of a treatment objective based on composite indices of activity, known as “treat-to-target” (t2t).4–6
Furthermore, early initiation of treatment with disease modifying anti rheumatic drugs (DMARDS) may slow down or prevent progression and also thereby modify associated excess risk. This “window of opportunity” hypothesis has been confirmed.7–9
The introduction of recent-onset arthritis disability (ROAD) departments in many countries is a direct response to the intention to use early treatment strategies to improve clinical and radiologic outcomes.10–13 Indeed, the hope that the course of the disease in its early stages may be reverted has promoted the use of intensive treatment strategies in patients even with undifferentiated arthritis.14,15 However, intensive treatment with DMARDS may also lead to increased toxicity levels.16 Verification of the characteristics related to a poorer disease course, at the beginning of the disease, may help to improve the risk/benefit ratio and adjust the application of an early and intensive therapeutic approach in cases with poorer prognosis.17
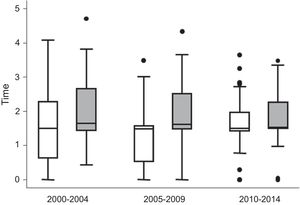
The establishment of TC y t2t strategies over the last 10 years has had unexpected consequences in biomarker research, precisely due to its great efficacy. Since patients with poorer prognosis are treated more intensively, the variables of standard outcome such as the consequence of remission or long term radiologic progression, show no differences between patients with poorer or better initial prognosis.18 What is more, in recent years, the Princesa Early Arthritis Register Longitudinal (PEARL) study detected that treatment intensity between positive and negative ACPA patients has gradually levelled out (Fig. 1), probably with the aim of taking advantage of the window of opportunity. To avoid this problem and explore poor prognosis biomarkers in patients with early stage arthritis different variable subrogates from severity have been proposed, which are related to the use of more intense treatment such as the need for the use of biological treatment (BT)19 or the accumulation of treatment.18,20 However, the validity and metric properties of these variables is yet to be determined.
Description of the treatment received by the patients of the PEARL cohort study during the 2000–2004, 2005–2009 and 2010–2014 periods. Differentiation of the variable time with disease-modifying anti-rheumatic drugs (DMARDS) between ACPA-positive patients (grey) and ACPA-negative (white) patients. Data are shown as the median of the variable DMARDS (line inside the box) and the percentiles 25.75 (lower and upper edges of the box), 10 and 90 (ends of the lines outside the box). The dots represent cases outside of this range.
Within this context, confronted by the need for a variable of valid severity which would be helpful in evaluating the usefulness of biomarkers in recent-onset arthritis, we decided to carry out an assessment of existing tools. To do this we conducted a systematic review of the literature aimed at: (1) identifying indices or tools which would allow us to discriminate between patients with serious and nonserious RA and (2) assessing their validity and applicability from existing data in the medical history. After this we explored whether the tools identified would serve to estimate the severity of patients with recent-onset arthritis at a sufficiently early enough time of follow-up to be able to carry out effective biomarker studies, and for this we established a 2-year post diagnosis cut-off point.
MethodsWe carried out a systematic review of studies on the development or validation of indices or variables of severity in adult patients with RA.
Search StrategiesA sensitive search in MEDLINE (1950–January 2017) was conducted by an experienced person (LC), using Mesh and free text terms, with no language restrictions. The strategy included synonyms for “rheumatoid arthritis”, “validation studies” or “development of (“variables” or “indices” or “questionnaires”) or “prognostic studies” and the term “severity”. The search strategy is available as in supplementary material.
Study SelectionStudy selection was made by a trained reviewer (ET) in 3 steps: (1) selection by titles; (2) selection by summaries and (3) compilation of complete text of the selected references and their assessment, making the final selection according to previously set criteria. A reason was recorded in order to exclude a study in all nonincluded cases.
Assessment of Index QualityThe indices or severity questionnaires found in this search process were analysed by using the check-list of Consensus-based Standards for the selection of health Measurement Instruments (COSMIN).21 This list contains standards for adjusting design requirements and statistical methods for studies on psychometric properties of health measurement instruments and may be used to determine whether a study meets with the standards of good methodological quality. It contains 4 steps for the calculation of methodological quality of the different aspects for the validation of the study measurement instrument.
Finally, in each of the variables of the indices we analysed the qualities we considered particularly relevant for the research group, such as clarity of definition, viability, presence in the first 2 years of evolution and domain to which they belonged.
ResultsIdentification of Tools for Assessment of SeverityThe search strategy identified 3519 articles (see Fig. 1 in the supplementary material), 3505 of which were excluded after reading the title and abstract. The reason for exclusion was mainly that there was no objective of validation or development of an index or questionnaire of severity in the RA. Of the remaining 14, another 7 were excluded for the same reason after a detailed reading (supplementary materials). Finally, 7 articles were included in the systematic review the objective of which was the development or validation of a severity index in RA. In total, 3 severity indices in RA were found, the Patient Activity Scale (PAS), the RARBIS and the CIRAS, the characteristics of which are described below.
Patient Activity ScaleThe patient activity scale (PAS) was developed by Wolfe et al.22 from score means from the Health Assessment Questionnaire (HAQ) (on a scale of 0–10), and 2 visual analogue scales, one for pain and the other for total activity; all variables were provided by the patient. This scale which was completed by the patient had demonstrated strong correlation with the disease activity in clinical trials. Wolfe et al. used them as synonyms for severity in the 2006 study23 and they tried to validate them by studying their capacity of discrimination for patients with different treatment loads.
The features of the 7541 patients included in the criteria validation study are presented in Table 1. In a similar fashion to other indices, no information of baseline variables typically associated with greater severity were included. The majority of patients (87%), on check-up, used a DMARD and/or biologic (74.2% used a DMARD, 33.1% a biologic and 18.3% did not use either). The mean PAS was 3.4. The highest score of the PAS index was reached in patients who had not taken any medication (PAS 3.7, interquartile range [IQR] 1.7–5.7) and in those who had not previously taken a DMARD or biologics (PAS 3.7, IQR 2.0–5.4).
Characteristics of the Indices Included in the Review.
| Index | Domains and variables | Range | Apparent validity | Validity and construct | Validity of criterion | Viability | Justifiable in the first 2 years |
|---|---|---|---|---|---|---|---|
| PAS | Pain (EVA) Function (HAQ) Severitya (EVA) | 0–10 | Obtained from core-set ACR components, not specifically linked to severity, but to activity. Little apparent validity COSMIN: + | Appropriate against construct activity correlation Strong with ACR20 and 50 and with DAS Less clear against severity COSMIN: + | AUC=0.70 to differentiate between treatment with DMARDS and biologics COSMIN: +++ | +++ All variables present | +++ (all variables present) |
| RARBIS | Surgery (C1–C2 fusion), any joint of the hand, foot, large joints (hips, knees, shoulders, elbows) Radiology (C1–C2 subluxation, Any erosion) Extra-articular symptoms (vasculitis, pulmonary nodules) Clinical (arthritis outbreak, overall evaluation of disease by physician, functional status, morning stiffness) Laboratory (FR>normality limit, VSG>age/2 or PCR>normality limit or platelets>450,000) | 0-15b | Developed from Delphi Appropriate apparent validity COSMIN: ++ | Appropriate COSMIN: ++ | Not assessed | +++ | ++ (difficult surgical and radiologic destruction variables) |
| CIRAS | Identification/personal data (age, gender) Analytical variables of inflammation (Number of inflammatory markers) Use of health services (visits to physiotherapist) Immunological variables Syndrome (FR) Extra-articular symptoms (Felty) Analytical variables of inflammation (number of platelets) Analytical biochemical variables (biochemical panels) | 6.5 | Developed from RARBIS. However, is formed by variables which reflect medical activity and may be “contaminated” with own measurements of the t2t Apparent validity doubtful COSMIN: + | Appropriate COSMIN: ++ | Not assessed | ++ | +++ |
Methodological quality according to COSMIN: +: low; ++: moderate; +++: excellent. Viability: +: few variables present; ++: quite a lot of variables present; +++: all variables present. Justifiable during the first 2 years: ++: quite a lot; +++: completely.
NA: not assessed.
The authors assessed the capacity of the treatment variables to predict the PAS score using the calculation of the area under the ROC (AUC) curve and the percentage of correct classification, obtaining a AUC of .64 and an accurate classification percentage of 60.5%. Wolfe et al. concluded that the use of demographic variables and treatment in administrative data bases did not enable patient groups to be distinguished according to their severity with appropriate sensitivity and/or specificity.
Rheumatoid Arthritis Records-Based Index of SeverityThe Rheumatoid Arthritis Records-Based Index of Severity (RARBIS) was conceived as a severity index of RA based on medical records. It uses a series of previously chosen severity indicators with Delphi methodology through a panel of 6 rheumatologist experts.24 The RARBIS includes 5 subscales or domains: surgery, radiology, extra-articular manifestations, clinical symptoms and laboratory variables. The treatment subscale was finally excluded from the total RARBIS. Those domains which had been assessed by the panel as being highly related to severity were awarded a higher score in the index.
The development of the index in a cohort of veterans25 was studied at a later date and the validity of construct through comparison of the RARBIS with treatment intensity explored: low (without DMARD or biological treatment), moderate (hydroxychloroquine, gold salts or sulfasalazine), high (metotrexate, azathioprine, leflunomide or cyclosporine) or very high (biological treatment), demonstrating correlation with the treatment intensity of RA (construct validity),25 although the association was weak (r=.35, 95% CI, .18–.55).
In a posterior study the convergent validity with regard to the DAS28.26 was established One hundred patients were selected for this from the cohort (Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study (BRASS) with a DAS28 equally distributed between 4 quartiles. Correlation between the total RARBIS and the DAS28 was weak (r=.41, 95% CI, .23–.56), and that of the different subscales or domains of the RARBIS index with the DAS28, which is some cases was nonexistent. However, the correlation improved when the authors increased the weight bias of the clinical subscale and reduced the weight bias of the surgical subscale (r=.48, 95% CI, .31–.62).21
Claims-based Index for Rheumatoid Arthritis SeverityThe same research group of the RARBIS subsequently developed the Claims-based Index for Rheumatoid Arthritis Severity (CIRAS),27 an index of RA severity based on potential indicators of severity of different domains, both demographic and clinical and, above all, of use in health services. For this, they included 120 patients from the before mentioned cohort of veterans from the New England VA Health System who had at least 2 recorded visits with a diagnosis of RA (CIE 9th edition, code 714.0), at least 2 hospital visits between July 1999 and June 2001, and sufficient entries in the medical record relating to the disease. Patient characteristics are recorded in Table 1.
The authors developed the Claims-based Index of Rheumatoid Arthritis Severity (CIRAS) index using lineal regression models, according to the different variables extracted from administrative data. They used several different selection procedures to achieve the best model, according to its R2. The final CIRAS included the administrative variables which are present in the best model. Each of the variables was weighted by its correlation coefficient. The construct validity was assessed using the correlation analysis the CIRAS and the RARBIS, obtaining a correlation coefficient between CIRAS and RARBIS index, obtaining rho=.56 between CIRAS and RARBIS with the medication subscale and a rho=.51 without the medication subscale. In a posterior study with 315 patients28 the convergent validity of the CIRAS was assessed with regard to the DAS28-PCR and the multidimensional HAQ (MD-HAQ). The correlation between the CIRAS and the DAS28-PCR (r=.07) and MD-HAQ (r=.008) was very low. Finally, the results of a lineal regression model with additional variables extracted from the administrative data of the Medicare cohort not included in the CIRAS (interstitial pulmonary involvement, hand surgery, request for tuberculin test, ACCP request, corticoids, opioids, nonsteroid anti-inflammatory drugs, number of biologic and nonbiologic drugs) demonstrated a low capacity for explaining the variability of the DAS28-PCR (R2=.23).
The variable description, domains and scores of the 3 indices are included in the review described in Table 1.
Critical Assessment of the IndicesThe aspects of validation studied, and the qualities considered to be a particular relevance by the research group are described for the 3 indexes in Table 1.
DiscussionThe search for prognostic factors or markers requires reliable outcomes. In diseases where the outcome variables are extremely objectives and consistent, such as cancer (death or tumour progression) or osteoporosis (appearance of new fracture or variation of bone mineral density) personalised medicine or at least medicine adjusted to patient profiles seems increasingly more viable. However, and at the moment, in RA ROAD we lack these objectives or consistent variables. The key gold standard which has been available for some years is radiologic progression and this has succumbed to intensive treatment strategies5 and the appearance of drugs which are able to stop the radiologic progression, even in patients without a minimum therapeutic response.
The aim of this paper was to identify composite severity indices, based on different domains, which could be applied to the search for predictive factors of severity in RA. The systematic search carried out up until 2017 only led to the identification of 3 tools, the PAS,22 RARBIS25 and CIRAS indices.27,29 They began development a short time after the beginnings of the biologic era in the field of rheumatology, approximately 15 years ago, and considerable effort is required to find an index which is able to calculate the severity of RA.
The oldest of them, RARBIS, is based on data obtained from medical files and its main defect is that, due to age, it weighs up circumstances such as the presence of the Felty syndrome, which is hardly ever currently seen in our patients. Some other variables included in this index, particularly the surgical and radiological domains are not present during the first 2 years of the disease. Lastly, its poor correlation with treatment needs or clinical activity of the patients led the authors to rethink their approach to the problem.
Wolfe et al.22 used the PAS as a severity variable without previously carrying out a validation study, they simply exchanged the concept of activity for severity. Neither did they obtain a good discrimination capacity for the criteria “treatment with DMARD and biologics”.
The CIRAS is essentially based on obtaining information on the use of electronically recorded health services. Although the variables included in this index may be collected during the first 2 years of evolution of patients with recent-onset arthritics, the inclusion of TC and t2t strategies in standard clinical practice means that the number of visits to the rheumatologist and the undertaking of many laboratory determinations do not have to be related to greater severity and do have to be related to the putting into practice of this therapeutic strategy. Furthermore, visits to the physiotherapist which were highly frequent in previous times, are exceptional in the biological era, thanks to better control of the disease activity during the first years of evolution. Moreover, the CIRAS index includes variables which are related to the use of the health services (request for inflammation markers, platelet count and other biochemical parameters) but allow the t2t strategy to be used without this necessarily involving increased severity. Other variables included, such as the Felty syndrome are exceptional in clinical presence.
Validation of these indices leads to several methodological problems. Although the development of the RARBIS was based on a panel of experts, the subscales (domains) and indicators (variables) will be weighted in accordance with the criteria of the panel depending on the correlation with severity. To assess the possible interdependence or relative contribution of the selected variables no item reduction technique was used which would prevent possible redundancies. In the CIRAS the variables included were chosen as part of the best model identified, not according to relevant aspects of construct, but in accordance with the availability of containment in health records, without wondering whether they reflected all the relevant aspects of the construct “severity”. In both indices only the validity of construct is contemplated (convergent validity), and no other aspects of validation or reliability are considered. Correlation with the construct used in both cases was weak, as previously explained. Moreover, due to the use of the same cohort of patients for the development of both indices, some correlations found were expected. The development of the PAS puts forward 2 major limitations, the first of these is the use of self-recording measures by the patient, which may lead to a bias in the assessment of severity; the second is that only the criteria validity was contemplated, with a low predictive capacity for seriously ill patients.
The recently published Scandinavian designed study NORD-STAR30 is the first independently randomised trial to compare treatment with synthetic DMARDS and 3 different lines of BT in a head to head analysis in a population with early RA. The aim of the study was to identify the best induction remission treatment, but also to improve the therapeutic optimisation strategies. Whilst waiting for the first results it puts into practice the before-mentioned concepts of t2t and TC, and fantasizes with the idea of carrying out an individualised treatment to obtain remission and once obtained, use the best therapeutic optimisation strategy. In patients with early RA, good continued response in a large amount of patients after suspension of anti TNF supports the idea of the window of opportunity.7–9 The classification of patients according to their severity and evaluation of the use of biomarkers thanks to this severity variable, brings us to the idea of personalised medicine which will allow us to chose the treatment in an individualised manner and indeed reduce direct and indirect costs derived from the disease and its treatment. To conclude, this review confirms that there is no index which calculates the severity of early RA in the 2 years of follow-up and which adapts to current strategy of therapeutic management. Due to all of the above, we believe it is now reasonable to develop a new ad hoc severity index for ROAD patients.
Ethical DisclosuresProtection of people and animalsThe authors declare that for this research no experiments have been carried out on humans or animals.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article
FinancingThis study was conducted thanks to the funds from projects RD12/0009/0017 and PI14/00442 of the Ministry of the Economy and Finance (Instituto de Salud Carlos III), co-financed by the European Fund for Regional Development (FEDER).
Conflict of InterestsDr. González-Álvaro declares he received financing from the Instituto de Salud Carlos III for the undertaking of this study. Moreover, independently from this study, he has received payments over the last 5 years for consultancy work or presentations from Abbvie, BMS, Lilly, UCB and Pfizer. Financing for research projects are not restricted to Roche and UCB; support for attendance to congresses by Abbvie, BMS, MSD, Pfizer and UCB. Lastly, patent PCT/ES2015/070182 has been presented and approved on a European level.
Loreto Carmona and Isidoro González-Álvaro share the senior authorship.
Please cite this article as: Toledano E, García de Yébenes MJ, González-Álvaro I, Carmona L. Índices de gravedad en la artritis reumatoide: una revisión sistemática. Reumatol Clin. 2019;15:146–151.