To study the frequency of different autoantibodies to extractable nuclear antigens (ENAs) in rheumatoid arthritis (RA) patients and to correlate findings with clinical manifestations, disease activity and radiological damage.
MethodsA total of 230 RA patients were included and 75 healthy controls. In all patients rheumatological assessment was done and routine laboratory investigations and immune profile were performed in both patients and controls, including: RF, ACPA, ANA and anti-ENAs (Ro/SSA, La/SSB, U1-RNP, anti-Jo-1 and anti-Sm). Radiological damage was scored using Sharp/van der Heijde, and disease activity was evaluated by DAS28-ESR and DAS28-CRP.
ResultsRF was positive in 101 (43.9%), ACPA in 220 (95.7%), ANA in 58 (25.2%), anti Ro in 31 (13.5%), anti-La in 10 (4.3%), anti-Jo1 in 5 (2.2%) and anti-RNP in 2 (0.9%). Anti-Ro/SSA positively correlated with sicca symptoms (p=.02), RF titer (p<.001), ANA (p<.001), DAS28-ESR (p=.026), and DAS28-CRP (p=.003). Anti-La antibodies correlated positively with SJC (p=.001), TJC (p=.001), ANA (p<.001), DAS-28 ESR (p=.007). Anti-Jo-1 correlated positively with interstitial lung disease (ILD) (p≤.001), RF titer (p=.037) and ANA (p≤.001). Anti-RNP antibodies correlated positively with disease duration (p≤.001), ACPA titer (p≤.001) and ANA (p=.014). In the controls ANA was positive in two (2.7%), anti-Ro in three (4%), and none of the controls tested positive for other autoantibodies.
ConclusionsIn RA patients, positive ANA is frequent and positively associated with anti-Ro, anti-La and anti-Jo1 autoantibodies. Screening for autoantibodies against other anti-ENAs seems mandatory in RA patients especially when ANA is positive. RA cases with positive Anti-Jo-1 may develop anti synthetase syndrome and ILD.
Estudiar la frecuencia de diferentes autoanticuerpos frente a antígenos nucleares extraíbles (ENA) en pacientes con artritis reumatoide (AR) y relacionar los hallazgos con las manifestaciones clínicas, la actividad de la enfermedad y el daño radiológico.
MétodosSe incluyeron un total de 230 pacientes con AR y 75 controles sanos. En todos los pacientes, la evaluación reumatológica, las investigaciones de laboratorio de rutina y el perfil inmune se realizaron tanto en pacientes como en controles, incluidos: RF, ACPA, ANA y anti-ENA (Ro/SSA, La/SSB, U1-RNP, anti-Jo-1 y anti-sm). El daño radiológico se puntuó con Sharp/van der Heijde y la actividad de la enfermedad se evaluó mediante DAS28-ESR y DAS28-CRP.
ResultadosLa RF fue positiva en 101 (43.9%), ACPA en 220 (95.7%), ANA en 58 (25.2%), anti Ro en 31 (13.5%), anti-La en 10 (4.3%), anti-Jo1 en 5 (2,2%) y anti-RNP en 2 (0,9%). Anti-Ro/SSA se correlacionó positivamente con los síntomas de sicca (p=.02), el título de RF (p<.001), ANA (p<.001), DAS28-ESR (p=.026) y DAS28-CRP (p=.003). Los anticuerpos anti-La se correlacionaron positivamente con SJC (p=.001), TJC (p=.001), ANA (p<.001), DAS-28 ESR (p=.007). El anti-Jo-1 se correlacionó positivamente con la enfermedad pulmonar intersticial (EPI) (p≤0,001), título de RF (p=0,037) y ANA (p≤0,001). Los anticuerpos anti-RNP se correlacionaron positivamente con la duración de la enfermedad (p≤0,001), el título de ACPA (p≤0,001) y ANA (p=0,014). En los controles, ANA fue positivo en dos (2.7%), anti-Ro en tres (4%) y ninguno de los controles dio positivo para otros autoanticuerpos.
ConclusionesEn pacientes con AR, el ANA positivo es frecuente y se asocia positivamente con autoanticuerpos anti-Ro, anti-La y anti-Jo1. La detección de autoanticuerpos contra otros anti-ENA parece obligatoria en los pacientes con AR, especialmente cuando la ANA es positiva. Los casos de AR con Anti-Jo-1 positivo pueden desarrollar el síndrome de sintetasa e ILD.
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by swelling, tenderness, progressive destruction of synovial lined joints, and progressive disability.1 RA is a disease with many faces, some of these correlate with the presence of certain autoantibodies for example anti Ro/SSA has an association with Sjögren's syndrome in RA.2–4 The presence of anti-Jo-1 in RA patients may indicate an overlap between RA and anti-synthetase syndrome (ASS) especially in anticitrullinated peptide/protein antibodies (ACPA) positive cases.5–11
Extractable nuclear antigens (ENAs) are autoantibodies that react with proteins in the cell nucleus, these proteins collectively termed “extractable” because they can be isolated from cell nuclei using saline. Autoantibodies directed to intracellular components are a characteristic feature of antinuclear antibody (ANA)-associated rheumatic diseases (AARD), like systemic lupus erythematosus (SLE), systemic sclerosis (SSc), mixed connective tissue disease (MCTD), primary Sjögren syndrome (pSS), and autoimmune inflammatory myositis (AIM). Some of these autoantibodies are highly specific for the individual AARD and included in the classification and/or diagnostic criteria for these autoimmune diseases.12
Autoantibodies against ENAs are best studied in SLE patients13–19 and may appear many years before SLE diagnosis.15,16 These autoantibodies to ENAs are associated with the pathogenesis of lupus nephritis and play important roles in the assessment of disease activity and/or severity.14
The finding of Anti-Jo-1 in RA patients11 was one of the reasons we decided to perform the current study examining the prevalence of Jo-1antibodies in a large series of RA patients, its clinical relevance and association with anti-Ro and anti-La and other ENA autoantibodies.11 This study may lead to better understanding and early diagnosis and management of comorbidities.
Antinuclear antibodies in RA have been studied repeatedly since 1961 when Weir et al.20 found, with little sensitive tests, that 14% of RA patients could be ANA positive. In the seventies more specific aspects based on an extractable nuclear antigen were studied. It was even suggested that ANA positive RA patients had a lupus like disease and should be treated differently, especially with corticosteroids.21 Many studies were performed since regarding ANA and RA nuclear antigen (RANA) in RA patients, especially in the 1980s, looking for correlations with for example rheumatoid factor, HLA factors, Epstein Barr virus.22–25
Only few studies have systematically investigated the different types of autoantibodies against ENA in a large series of RA patients and correlating the findings with clinical manifestations. The aim of the current study was to examine the prevalence of autoantibodies against ENAs (Ro/SSA, La/SSB, UI-RNP, Jo-1 and anti-Sm) in a cohort of RA patients and to correlate the presence of these autoantibodies with different clinical manifestations, disease activity indices and radiological damage.
Patients and methodsClinical assessmentA total of 230 consecutive RA patients (females 177 (77%) and males 53 (23%)) could be included in the study, who visited the rheumatology clinics of the Rheumatology and Rehabilitation Department, Dr. Erfan and Bagedo General Hospital, Jeddah, Saudi Arabia. All fulfilled the 2010 ACR/EULAR classification criteria for RA.26 Detailed rheumatological history and physical examination was carried out in all patients including the clinical variables: swollen joint count (SJC), tender joint count (TJC), and duration of morning stiffness in minutes. Also symptoms of Sjögren's syndrome were studied and relevant pulmonary symptoms. Special attention was paid during clinical assessment to carefully exclude any symptoms or signs suggestive of these disorders, e.g. Raynaud's disease, swollen puffy fingers, proximal muscle pain or tenderness, Gottron's sign, telangiectasia, sclerodactyly and subcutaneous calcifications, because of the known associations of these syndromes with anti-ENA autoantibodies tested.
The study was performed in close cooperation with the departments of rheumatology, and internal medicine in the universities of Cairo, Assiut and Ismaila in Egypt. All patients gave informed written consent to be enrolled into the study according to the Declaration of Helsinki.
As controls 75 healthy subjects were also included matched for age and sex with the RA group of patients. They were 57 (76%) females and 18 (24%) males; their mean age in years (SD) was 44.87±(7.74). All control subjects had no previous history of any joint swelling and with no symptoms or signs suggestive of autoimmune disease and negative family history of RA or other autoimmune disease.
Laboratory investigationsBaseline routine laboratory at inclusion for the RA group were: erythrocyte sedimentation rate (ESR) first hour (mm/h), C-reactive protein (CRP) levels (mg/dl), complete blood count, complete liver and kidney function tests, CPK and urine analysis.
Rheumatoid factor (RF) and ACPA were tested in all patients. Serum samples were collected and stored at −80°C. ACPA (IgG) were measured using commercially available second-generation ELISA kits (DiastatTM Axis, Shield Diagnostics, Dundee, Scotland). ACPA assay was performed according to the manufacturer's instructions, and values were expressed in units per millilitre (U/ml) and were considered to be positive at a cut-off value of more than 5U/ml. The IgM-RF was assayed using a quantitative immunonephelometric test for RF and was considered positive when values were higher than the cut-off value of the kit (15U/ml).
Anti-extractable nuclear antigens (ENA) assayENA autoantibodies as well as ANA were tested for both the RA group of patients and the normal healthy control subjects for effective validation of the immunological laboratory findings. The following ENA autoantibodies to Smith (anti-Sm), ribonucleoproteins (anti-U1-RNP), Ro/SSA (anti-Ro/SSA), SSB/La (anti-La/SSB) and anti-Jo-1 were assessed using Alegria® assay which features bar-coded 8-well-microstrips, called Alegria® Test Strips. The Alegria® test strip holds a complete set of reagents including enzyme conjugate, enzyme substrate, sample buffer and a test specific control. The determination is based on an indirect enzyme linked immune reaction with the following steps: antibodies present in positive samples bind to the antigen coated on the surface of the two reaction wells forming an antibody antigen complex. After incubation, a first washing step removes unbound and unspecific bound molecules. Subsequently added enzyme conjugate binds to the immobilized antibody–antigen complex. After incubation, a second washing step removes unbound enzyme conjugate. Addition of enzyme substrate solution results in hydrolysation and color development during incubation. The intensity of the blue color correlates with the concentration of the antibody–antigen-complex and can be measured photometrically at 650nm. The calculation range of this assay is 0–200U/ml [normal <15U/ml, borderline 15–25U/ml, elevated >25U/ml].
Radiologic assessmentStandard X-rays of hands and wrists and of forefeet were made. Joint damage was assessed for all patients at the time of their inclusion in the study by the simplified radiological Sharp/Vander Heijde score (SHS).27
Statistical analysisAnalysis of data was performed with the statistical package for the social sciences (SPSS) version 15. As disease duration and most continuous laboratory and disease activity scores were positively skewed, these values were log-transformed (after adding 1 to eliminate zero values) to normalize their distribution before statistical analysis. Univariate Pearson's correlations were computed between ENAs and other clinical variables and disease activity indices (p values <.05 are accepted as statistically significant).
EthicsThe local ethical committee approved the study design. All patients and controls gave informed written consent to be enrolled into the study according to the Declaration of Helsinki.
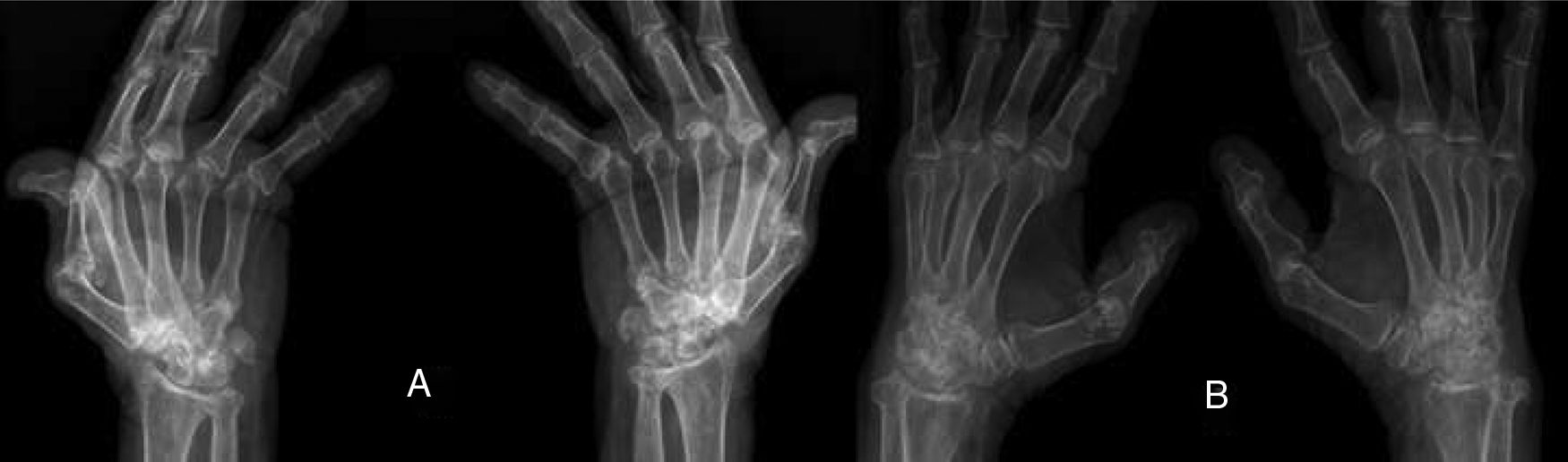
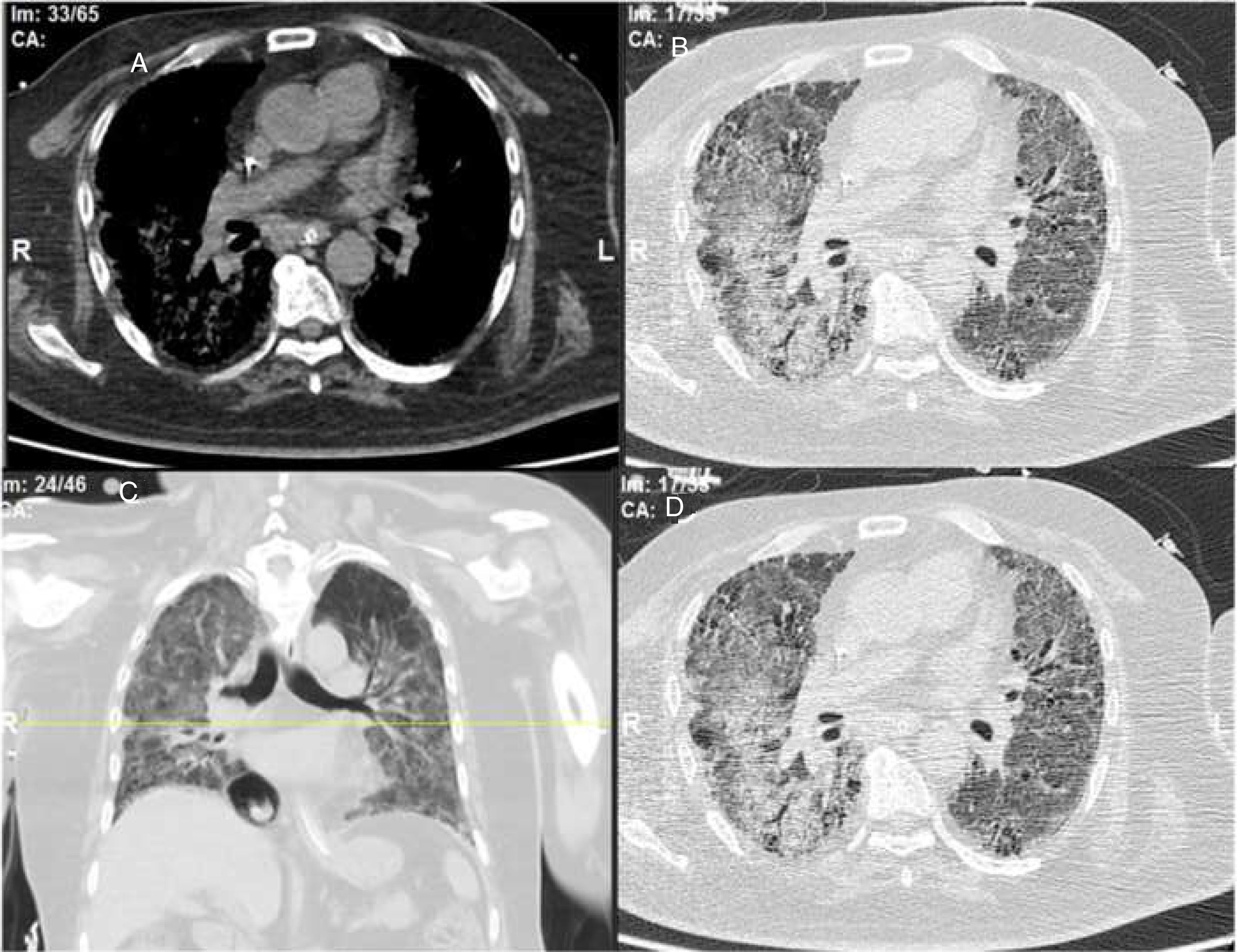
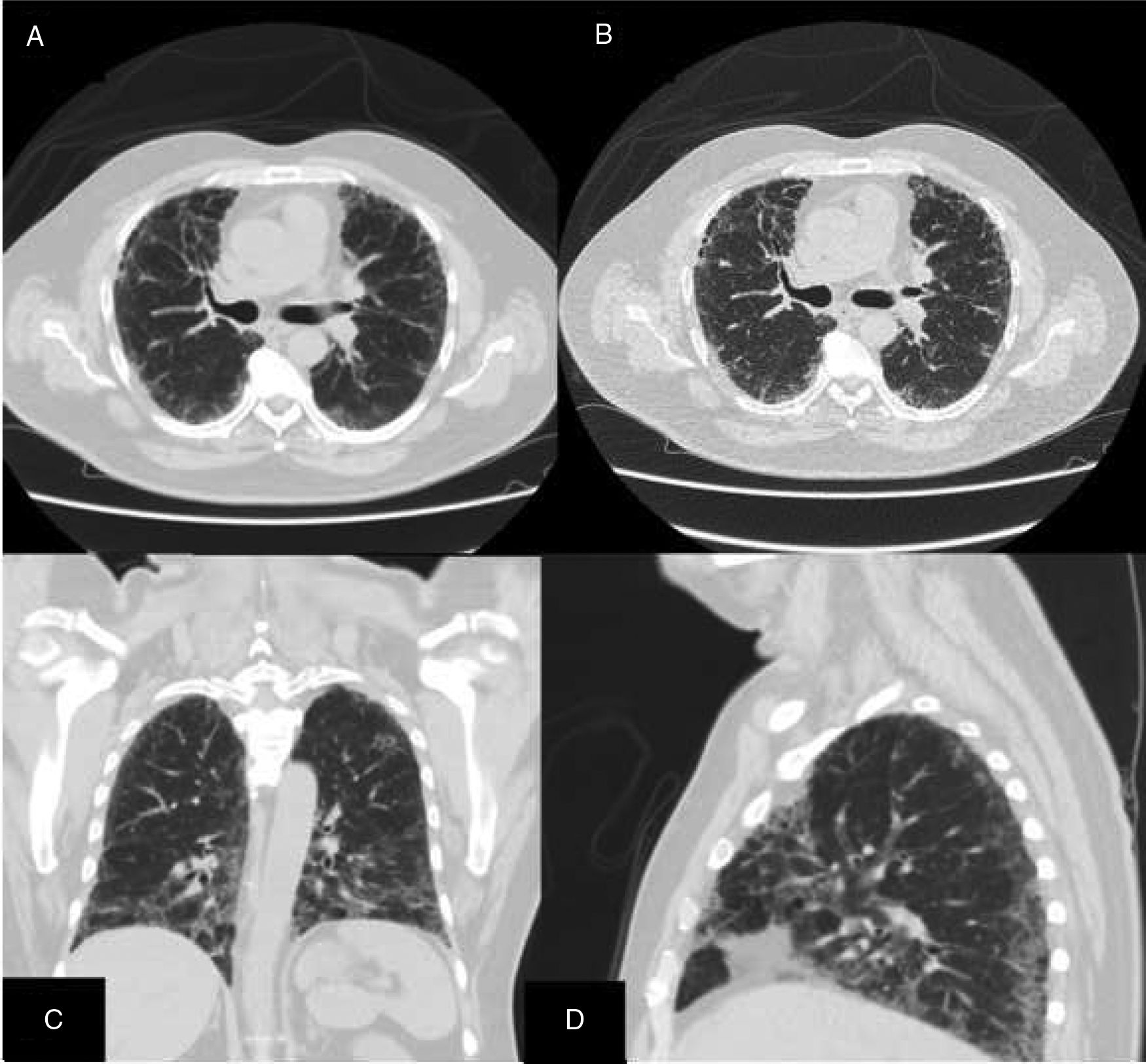
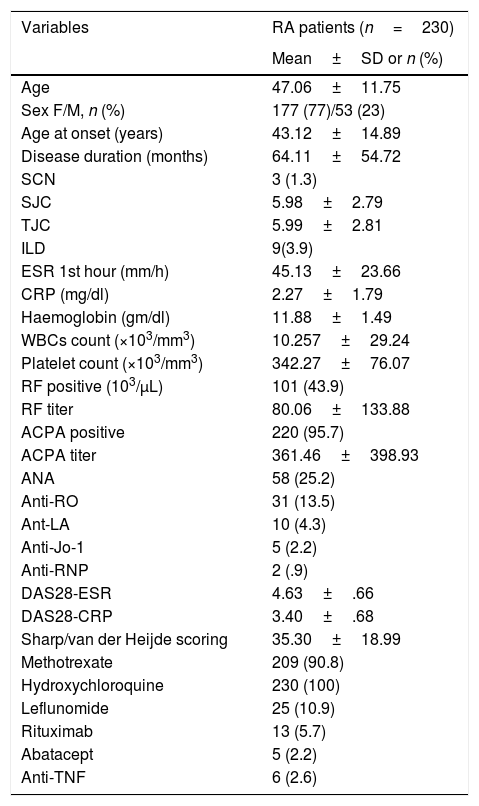
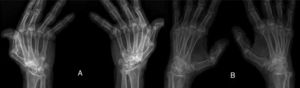
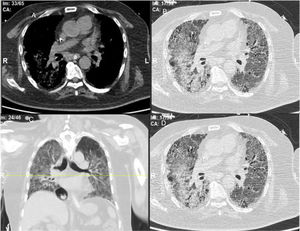
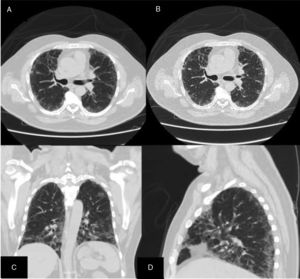
ResultsWe could include 230 patients (female 177 (77%) and males 53 (23%)) with established RA, fulfilling the 2010 ACR/EULAR classification criteria for RA.12 Their mean age was 47.06±11.75 years and disease duration 64.11±54.72 months. RF was positive in 101 (43.9%) and ACPA was positive in 220 (95.7%), while ANA was positive in 58 (25.2%), anti-Ro/SSA in 31 (13.5%), anti-La/SSB in 10 (4.3%), anti-RNP in 2 (.9%) and none of the patients showed anti-Sm antibodies. Anti-Jo-1 was positive in 5 (2.2%), none of them had increased CPK levels and all five cases showed typical erosive changes consistent with the diagnosis of RA (Fig. 1). Two out of the 5 cases with positive Anti-Jo-1 had ILD (Figs. 2 and 3). Detailed demographic features, clinical manifestations, laboratory investigations, SHS, drug therapy and disease activity scores are summarized in Table 1. In the controls (n=75), RF (low titer) was positive in 4, ANA was positive in two (2.7%), anti-Ro/SSA in three (4%), and none of the control group tested positive for anti-La/SSB, anti-Sm, anti-Jo-1, anti- U1RNP autoantibodies and none tested positive for ACPA.
(A) First RA case with anti-Jo-1 positive and showing the typical radiological features of RA in the form of intercarpaljoints, radio-carpal, and carpometacarpal joints erosions PIPs and MCPs erosions with juxta-articular osteoporosis and decreased joint spaces, MCPs subluxations and Z thumb deformity; (B) Second case with RA, and positive anti-Jo-1 showing intercarpal, radio-carpal, and carpometacarpal joints erosions, PIPs and MCP jointserosions with juxta-articular osteoporosis and joint spaces narrowing, consistent with the diagnosis of RA.
Demographic features, disease characteristics, disease activity indices and laboratory findings among the studied group of patients.
| Variables | RA patients (n=230) |
|---|---|
| Mean±SD or n (%) | |
| Age | 47.06±11.75 |
| Sex F/M, n (%) | 177 (77)/53 (23) |
| Age at onset (years) | 43.12±14.89 |
| Disease duration (months) | 64.11±54.72 |
| SCN | 3 (1.3) |
| SJC | 5.98±2.79 |
| TJC | 5.99±2.81 |
| ILD | 9(3.9) |
| ESR 1st hour (mm/h) | 45.13±23.66 |
| CRP (mg/dl) | 2.27±1.79 |
| Haemoglobin (gm/dl) | 11.88±1.49 |
| WBCs count (×103/mm3) | 10.257±29.24 |
| Platelet count (×103/mm3) | 342.27±76.07 |
| RF positive (103/μL) | 101 (43.9) |
| RF titer | 80.06±133.88 |
| ACPA positive | 220 (95.7) |
| ACPA titer | 361.46±398.93 |
| ANA | 58 (25.2) |
| Anti-RO | 31 (13.5) |
| Ant-LA | 10 (4.3) |
| Anti-Jo-1 | 5 (2.2) |
| Anti-RNP | 2 (.9) |
| DAS28-ESR | 4.63±.66 |
| DAS28-CRP | 3.40±.68 |
| Sharp/van der Heijde scoring | 35.30±18.99 |
| Methotrexate | 209 (90.8) |
| Hydroxychloroquine | 230 (100) |
| Leflunomide | 25 (10.9) |
| Rituximab | 13 (5.7) |
| Abatacept | 5 (2.2) |
| Anti-TNF | 6 (2.6) |
Rheumatoid arthritis (RA); subcutaneous nodules (SCN); swollen joint count (SJC); tender joint count (TJC); interstitial lung disease (ILD); erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); white blood cells (WBCs); rheumatoid factor (RF); anticitrullinated peptide/protein antibodies (ACPA); disease activity score (DAS); subcutaneous nodules (SCN); rheumatoid factor (RF); anti-cyclic citrullinated peptide (anti-CCP).
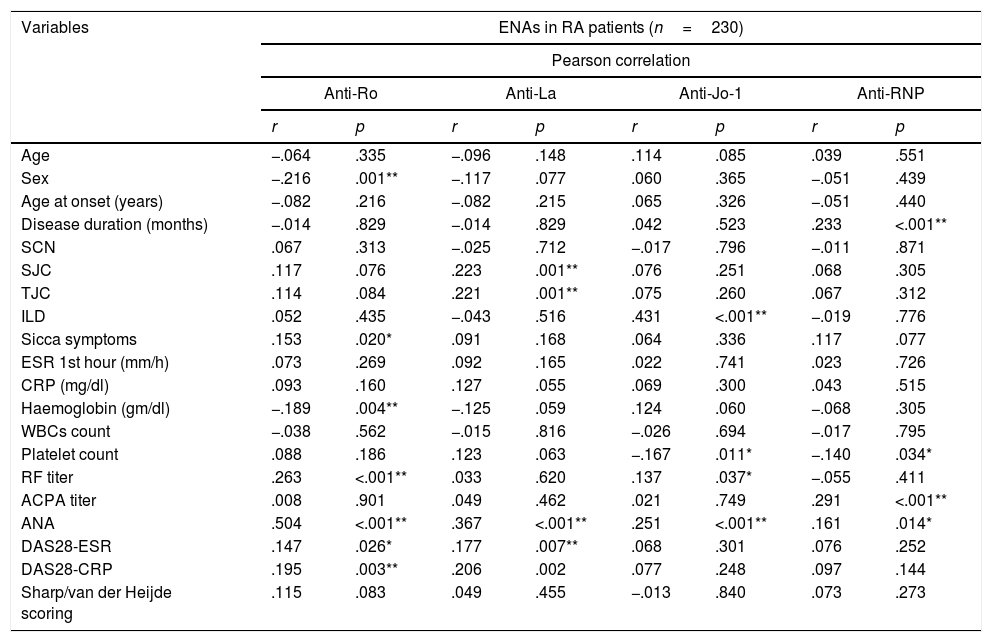
Correlation coefficients between different autoantibodies against ENAs (Ro/SSA, La/SSB, U1RNP and Jo-1) and various demographic features, clinical manifestations, laboratory investigations, radiological and disease activity scores are detailed in Table 2.
Correlation between extractable nuclear antigens and clinical features, laboratory findings and disease activity indices in RA patients.
| Variables | ENAs in RA patients (n=230) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pearson correlation | ||||||||
| Anti-Ro | Anti-La | Anti-Jo-1 | Anti-RNP | |||||
| r | p | r | p | r | p | r | p | |
| Age | −.064 | .335 | −.096 | .148 | .114 | .085 | .039 | .551 |
| Sex | −.216 | .001** | −.117 | .077 | .060 | .365 | −.051 | .439 |
| Age at onset (years) | −.082 | .216 | −.082 | .215 | .065 | .326 | −.051 | .440 |
| Disease duration (months) | −.014 | .829 | −.014 | .829 | .042 | .523 | .233 | <.001** |
| SCN | .067 | .313 | −.025 | .712 | −.017 | .796 | −.011 | .871 |
| SJC | .117 | .076 | .223 | .001** | .076 | .251 | .068 | .305 |
| TJC | .114 | .084 | .221 | .001** | .075 | .260 | .067 | .312 |
| ILD | .052 | .435 | −.043 | .516 | .431 | <.001** | −.019 | .776 |
| Sicca symptoms | .153 | .020* | .091 | .168 | .064 | .336 | .117 | .077 |
| ESR 1st hour (mm/h) | .073 | .269 | .092 | .165 | .022 | .741 | .023 | .726 |
| CRP (mg/dl) | .093 | .160 | .127 | .055 | .069 | .300 | .043 | .515 |
| Haemoglobin (gm/dl) | −.189 | .004** | −.125 | .059 | .124 | .060 | −.068 | .305 |
| WBCs count | −.038 | .562 | −.015 | .816 | −.026 | .694 | −.017 | .795 |
| Platelet count | .088 | .186 | .123 | .063 | −.167 | .011* | −.140 | .034* |
| RF titer | .263 | <.001** | .033 | .620 | .137 | .037* | −.055 | .411 |
| ACPA titer | .008 | .901 | .049 | .462 | .021 | .749 | .291 | <.001** |
| ANA | .504 | <.001** | .367 | <.001** | .251 | <.001** | .161 | .014* |
| DAS28-ESR | .147 | .026* | .177 | .007** | .068 | .301 | .076 | .252 |
| DAS28-CRP | .195 | .003** | .206 | .002 | .077 | .248 | .097 | .144 |
| Sharp/van der Heijde scoring | .115 | .083 | .049 | .455 | −.013 | .840 | .073 | .273 |
Rheumatoid arthritis (RA); subcutaneous nodules (SCN); swollen joint count (SJC); tender joint count (TJC); interstitial lung disease (ILD); erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); white blood cells (WBCs); rheumatoid factor (RF); anticitrullinated peptide/protein antibodies (ACPA); antinuclear antibody (ANA); disease activity score (DAS).
All anti-Ro/SSA positive cases were females, there was a positive correlation with sicca symptoms (r=.15, p=.02), RF titer (r=.263, p<.001), ANA (r=.504, p<.001), DAS28-ESR (r=.15, p=.026), and DAS28-CRP (r=.195, p=.003). Anti-Ro/SSA correlated negatively with sex (r=−.216, p=.001) being more frequent in female patients and significant negative correlation with haemoglobin level (r=−.189, p=.004). While Anti-La/SSB antibodies correlated positively with SJC (r=.223, p=.001), tender joint count TJC (r=.221, p=.001), ANA (r=.367, p<.001), DAS-28-ESR (r=.177, p=.007). Regarding anti-Jo-1 antibodies, a significant moderate positive correlation was found with ILD (r=−.431, p≤.001), RF titer (r=.137, p=.037), ANA (r=.251, p≤.001) and a negative association was found with platelets count (r=−.167, p=.011).
Anti-RNP antibodies were positively associated with disease duration (r=.233, p≤.001), ACPA titer (r=.291, p≤.001) and ANA (r=.161, p=.014) and correlated negatively with platelets count (r=−.140, p=.034). Furthermore anti-Ro/SSA positively correlated with anti-La/SSB (r=.478, p≤.001), and no significant correlation was found between anti-Ro/SSA versus anti-Jo-1 (r=.028, p=.668), and versus anti-RNP (r=−.037, p=.577). While anti-La/SSB showed no significant correlation with neither anti-Jo-1 (r=−.032, p=.632) nor anti-RNP (r=−.020, p=.763). Anti-Jo-1 showed no significant correlations with anti-RNP (r=−.014, p=.833). In this series ANA showed no positive correlations with biologic therapy (r=.005, p=.938). Furthermore no other associations were found between use of biological therapy versus other tested anti-ENA autoantibodies: anti-Ro/SSA (p=.618), anti-La/SSB (p=.905), Anti-Jo-1 (p=.106) and anti-RNP (p=.669). Moreover no significant association was observed between MTX dose and ILD in our study (p=.701).
DiscussionThe current study was carried out to examine the prevalence of autoantibodies against ENAs in a cohort of RA patients and to examine its association with other laboratory findings notably, RF, ACPA antibodies, ANA and other clinical and disease characteristics. In our study, RF was positive in 101 (43.9%) cases and ACPA was positive in 220 (95.7%), which is comparable with a previous study in a different series of early RA cases.28 An established fact is that ACPA is more sensitive than RF and may appear much earlier in the course of RA. Moreover positive ACPA can predict future development of RA in early undifferentiated arthritis,28 and play other diagnostic roles in patients with clinically suspected inflammatory arthralgia.1 In palindromic rheumatism (PR), hand joint involvement and positive ACPA are good predictors for RA development after 1 year of follow-up.29 Given that tight control strategy in PR by early use of DMARDs may control PR and prevent disease progression to RA.30
In the current study, ANA was positive in 58 (25.2%), anti-Ro/SSA in 31 (13.5%), anti-La/SSB in 10 (4.3%), anti-Jo-1 in 5 (2.2%), anti-RNP in 2 (.9%). Although a higher prevalence of ANA was observed in our study none of our patients showed any symptoms or signs suggestive of antinuclear antibody (ANA)-associated rheumatic diseases (AARD) as detailed in the methods section and none of the patients tested positive for anti-Sm which is quite specific for SLE diagnosis. In our study ANA showed no positive association with anti-TNF therapy (r=.005, p=.938). Furthermore no other associations were found between use of biological therapy versus other tested anti-ENA autoantibodies anti-Ro/SSA (p=.618), anti-La/SSB (p=.905), Anti-Jo-1 (p=.106) and anti-RNP (p=.669). Moreover no significant association was observed between MTX dose and ILD in our study (p=.701). Comparable to our findings and in the most recent work higher prevalence of ANA was observed in Mexican patients with early RA reaching 25.8% in control individuals.31
Anti-Ro/SSA and anti-La/SSB autoantibodies recognize different epitopes on small RNAs associated polypeptides. At least two major proteins are identified called Ro/SSA: a 52 kDa (Ro) and a 60kDa (Ro), while for La/SSB only one protein with 48kDa.3 Important to note is that anti-Ro/SSA antibodies can be detected in the sera of 30% of patients with SLE, even during the preclinical phase (latent lupus). In previous studies anti-Ro/SSA was strongly associated (90%) with some subgroups of SLE such as old-onset (>50y), and in patients with hereditary deficiency of complements components C2 or C4 or C1q with lupus-like syndrome, pSS (50–60%), undifferentiated connective tissue disease (UCTD),23 and also in RA patients with different percentages.2–4
In the current study, anti-Ro/SSA correlated positively with Sjögren's syndrome and its associated sicca symptoms (p=.02), RF titer (p<.001) and ANA (p<.001). Consistent with our findings, Skopouli et al.2 observed that positive anti-Ro/SSA antibodies seem to characterize a distinct group of RA patients who are exclusively females, and with high prevalence of sicca symptoms, and more frequent positive RF and ANAs when compared to those RA patients with negative anti-Ro/SSA autoantibody. In contrast Zanlorenzi et al.3 studied a total 385 with RA and found no significant associations between positive anti-Ro/SSA versus gender, DAS-28, functional classification, extra-articular features, positive RF, and positive ACPA. While Cavazzana et al.3 reported that anti-Ro/SSA positive RA patients presented a peculiar clinical picture characterized by extra-articular features notably amyloidosis, and episcleritis.
In our study anti-La/SSB antibody was positive in 10 (4.3%) patients and positively associated with anti-Ro/SSA (p≤.001), anti-JO-1 and ANA. Recently Jardel et al.32 evaluated the sera of 1173 patients with suspected UCTD for anti-Ro/SSA and/or anti-La/SSB autoantibodies. They identified 84 patients (13.5%) with isolated positive anti-Ro/SSA, out of these 75 patients with known clinical data, 15 (20%)were diagnosed with a definitive CTD including: SLE (5%, n=4), RA (5%, n=4), AIM (3%, n=2), pSS (1%, n=1), SSc (1%, n=1), UCTD (3%, n=2), and mixed CTD (1%, n=1). The authors concluded that positive anti-La/SSB, without anti-Ro/SSA autoantibody was not associated with CTD. In our study RA patients showed a positive association between anti-Ro/SSA and anti-La/SSB and only anti-Ro/SSA was significantly and positively correlated with sicca symptoms.
An interesting finding in our study is the positive anti-Jo-1 in five (2.2%) patients, and two of them had aggressive ILD who can be classified as RA-ASS overlapping syndrome. Anti-Jo-1 correlated positively with ILD and RF titer. One patient had a high RF titer, negative ACPA and positive ANA, erosions typical for RA, positiveanti-Jo-1, while the other case was seropositive for RF, ACPA and ANA, typical erosions consistent with the diagnosis of RA and concomitant ILD and we classified the two cases with ILD as having RA-ASS overlap. Methotrexate (MTX) induced hypersensitivity pneumonitis cannot explain acute respiratory distress syndrome as observed in two of our patients with RA-ASS overlapping syndrome. First MTX induced hypersensitivity pneumonitis usually occurs in the initial weeks to months of starting treatment with MTX as acute drug reaction33; second no significant association was observed between MTX dose and ILD in our study (p=.701); third and most important it is not known that MTX can induce positive anti-Jo-1 autoantibodies which are now considered the hallmark diagnostic autoantibody in ASS.
ASS is an uncommon multisystem CTD characterized by the presence of circulating anti-aminoacyl t-RNA synthetase antibodies and ILD which is considered a constant finding in this domain, often with AIM and erosive polyarthritis.34 Atypical features may include hand calcinosis and/or absent myositis.35,36 In previous studies was shown that ACPA-positive ASS patients may show overlapping features of RA-ASS and may develop refractory erosive arthritis.5,36 Only a few case reports describe ASS complicating the course of RA.5–11
In general, there is little justification for requesting tests for anti-ENA autoantibodies unless the ANA test is positive. Previously was recommended to perform an initial ANA screening test followed by confirmatory anti-ENA and anti-dsDNA antibody, if needed.37 Aggarwal et al.38 recently explained that a negative ANA does not indicate necessarily negative AIM associated autoantibodies (e.g. anti-Jo-1 and other antisynthetase autoantibodies) as a screening if ASS is clinically suspected. In our study none of our RA patients with positive antiJo-1 (n=5) showed clinical features of myositis and all had normal CK levels. The latter finding was supported by those of Aggarwal et al.38 who pointed out that ILD can be associated with ASS related autoantibodies, even in the absence of clinically apparent AIM. Although RA and ASS are distinct clinical syndromes, their co-occurrence may be rarely encountered and by early diagnosis serious or even fatal ILD can be prevented by early and appropriate treatment.5–10
A limitation of our study is that many studies were done regarding ENA autoantibodies including ANA, anti-Ro and anti-La in relation to secondary Sjogren's syndrome. The strength of our study is that only few studies have systematically investigated the different types of autoantibodies against ENA in a large series of RA patients correlating the findings with clinical manifestations. To our knowledge such type of study was not done before in Egypt or other Middle Eastern countries.
A further strength is that we studied the whole set of ENA autoantibodies and also assessed its relation to ANA and other diagnostic markers of RA including both RF and anti-CCP antibodies. We also raised important issues that negative ANA, does not always indicate that other anti-ENA autoantibodies are necessarily negative and we should change our way of thinking about this clinically relevant issue. If anti-Jo1 is positive in established RA patient, extra attention should be paid for any new onset of chest symptoms to avoid serious consequences.
Conclusions and recommendationsThis study showed that ANA is frequently positive (25.2%) in RA patients with a much higher figure than in controls. Negative ANA in RA patients does not indicate negative anti-ENAs autoantibodies as also shown in AIM29 and anti-Jo1 should be ordered in case of RA patients with explosive and rapidly progressive ILD’. Auto-antibodies against ENA are important and should be determined at least once in RA patients at disease onset. ASS is a rare systemic CTD and may complicate the course of RA, and rheumatologists should be aware of this rare under recognized clinical entity, especially in established RA patients with acute onset of respiratory distress and rapidly progressive ILD.
Conflict of interestNone of the authors has any conflict of interest regarding this study.
We thank the patients for their cooperation in the study. Mr Chris Hemke for translating the abstract in Spanish.