To describe practice patterns, long-term outcome, and related factors, in relation to biological therapies tapering in rheumatoid arthritis (RA) patients in a well-controlled real-world setting.
MethodsAn observational longitudinal retrospective 10-year study was conducted in all RA patients receiving biological agents in an RA clinic from May 2003 to October 2013. Biological treatment of patients with sustained DAS28<3.2 or SDAI<11 was tapered (dose down-titrated or interval widen) or discontinued as per practice protocol. Primary outcome of tapering was relapse, defined as an increase in DAS28≥1.2. Descriptive, survival analysis, and logistic regression analysis with first relapse as dependent variable were carried out.
ResultsOf 193 RA patients on biological treatment (mean age 54±14 years, 81% women), tapering was applied in 106 (55%) and discontinuation in 34 (17.6%). During follow-up 38 patients relapsed (62%). Rate of relapse was 10% at 6 months, 19% at 12 months, 33.2% at 2 years and 50% after 5 years. Mean time in dose reduction was 4.5 years [95% confidence interval (95% CI): 3.7–5.3]. Six patients (15.7%) did not respond after reinstatement of full dose of biologic. In the multivariate analysis, pain [OR=1.26 (95% CI: 1.11–1.43); P<.001] and erythrocyte sedimentation rate (ESR) [OR=1.01 (95% CI: 1.00–1.03); P=.011] at baseline were associated with relapse after tapering.
ConclusionsTapering may be considered a long-term option in RA patients on biologics and low disease activity, especially if low ESR and pain scores are present at baseline; treatment reinstatement could be considered a safe option in case of relapse.
Describir los patrones de práctica clínica, los resultados a largo plazo y los factores relacionados en relación a la optimización de las terapias biológicas en pacientes con artritis reumatoide (AR) en un entorno de vida real bien controlado.
MétodosSe realizó un estudio retrospectivo observacional longitudinal de 10 años que incluyó a todos los pacientes con AR que recibieron agentes biológicos en una consulta monográfica de AR entre mayo de 2003 y octubre de 2013. Se optimizó el tratamiento biológico (ajuste de dosis o ampliación de intervalo) en los pacientes con DAS28<3,2 o SDAI<11 de forma mantenida según un protocolo de práctica clínica. La variable principal fue la recaída, definida como un aumento en el DAS28≥1,2. Se realizó un análisis descriptivo, de supervivencia y modelos de regresión logística con la primera recaída como variable dependiente.
ResultadosDe 193 pacientes con AR en tratamiento biológico (edad media 54±14 años, 81% mujeres), se optimizó la dosis en 106 (55%) y se interrumpió el tratamiento en 34 (17,6%). Durante el seguimiento 38 pacientes recayeron (62%). La tasa de recaída fue del 10% a los 6 meses, del 19% a los 12 meses, del 33,2% a los 2 años y del 50% a los 5 años. El tiempo medio con dosis reducida fue de 4 años y medio (intervalo de confianza del 95% [IC 95%]: 3,7 a 5,3). Seis pacientes (15,7%) no respondieron después de restablecer la dosis completa de biológico. En el análisis multivariado, el dolor (OR=1,26 [IC 95%: 1,11 a 1,43]; p<0,001) y la velocidad de sedimentación globular (VSG) (OR por mm/h=1,01 [IC 95%: 1,00 a 1,03]; p=0,011) al inicio del estudio se asociaron a recaída tras la optimización.
ConclusionesLa optimización de la dosis se puede considerar una opción a largo plazo en pacientes con AR en tratamiento con agentes biológicos y baja actividad de la enfermedad, especialmente si la VSG y el dolor están en niveles bajos; la reinstauración del tratamiento podría considerarse una opción segura en caso de recaída en la mayoría de los pacientes.
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease, which can cause cartilage and bone damage, as well as disability. If inadequately treated, RA can lead to permanent joint damage and deformity.1 The availability of biological treatments that directly target components of the RA inflammatory cascade transformed the management of RA over the past 20 years resulting in a substantial improvement of outcome.2 This was accomplished thanks to numerous drug developments, as well as by implementation of early aggressive and dynamic treatment protocols based on adjustment until low disease activity (LDA) or remission is achieved.3 However, the use of biologic drugs is not exempt from risks and increased costs.4
Nowadays, the number of patients achieving remission or LDA is increasing, raising the question of whether drug-free remission would be possible.5 In addition, with RA prognosis improving, it appears that the continuation of disease-modifying anti-rheumatic drugs (DMARD) could exceed the risks of low-active RA, in terms of risk of serious infections and high maintenance costs. Indeed, tapering RA therapies has become a widespread practice to the point it has been included in the European League against Rheumatism (EULAR) 2013 guidelines for the management of RA.6 Despite some clinical trials have shown efficacy with low doses of different biologics,7,8 questions remain open as to how long the effect will last, whether reintroducing standard doses will be efficacious, or whether discontinuing biological treatment will be an option, and in whom.
The purpose of our study was to contribute to the description of the outcome of tapering and discontinuation in real-world settings. Concretely, we aimed to describe tapering of biological therapy in RA patients in our center, to describe how long could our patients remain on tapered doses or even discontinued treatment before a relapse, and to analyze factors that could predict relapse after tapering.
MethodsAn observational longitudinal retrospective study was conducted in our center. All adult patients diagnosed of RA according to ACR 1987 or ACR EULAR 2010 criteria, who started a biological treatment from 2003 to 2013 were identified. In our center, a protocol in place oblige us to collect standardized clinical data 6-monthly in patients on biological treatment. Data include: disease activity by the DAS28 and SDAI, dates of treatment start and modifications, and of events—comorbidity, with an emphasis on cardiovascular risk factors, and drug toxicity. In addition, as of treatment initiation the following are noted: rheumatoid factor (RF) and anti-citrullinated peptide antibody (ACPA) status, and radiographic damage (erosions).
By protocol, patients are eligible to have biologic treatment tapered if they achieve a stable state for longer than 6 months with DAS28<3.2 or SDAI<11. Tapering regimen depends on treatment: if the patient is on adalimumab (ADA), dose interval is widen from 2 to 4 weeks; if on etanercept (ETN), dose interval is widen from 7 to 14 days; if on infliximab (IFX), dose interval is widen from 8 to 12 weeks; if on tocilizumab (TCZ) dose is reduced from 8 to 4mg/kg; and if on rituximab (RTX) dose interval is widen from 12 to 36 weeks.
For this study, the primary outcome was first occurrence of relapse, defined as an increase in DAS28≥1.2 detected at any 6-monthly visit. Time to relapse was defined as the time from the first visit in which DAS28 was below 3.2 and the visit in which an increase of 1.2 over the baseline was observed.
Statistical AnalysisPatients and treatments are described by frequencies or means and standards deviations of descriptive variables. Kaplan–Meier curves were constructed to evaluate relapse of patients whose treatments were tapered because of LDA or remission, and are reported as cumulative failure at specific time-points. Observation spans from tapering visit to the visit in which relapse (failure variable) is noted or to the last follow-up visit in case no relapse occurred. Bivariate and multivariate logistic regression analysis with stepwise modeling was performed to identify predictors of relapse. A P-value of .05 was considered significant.
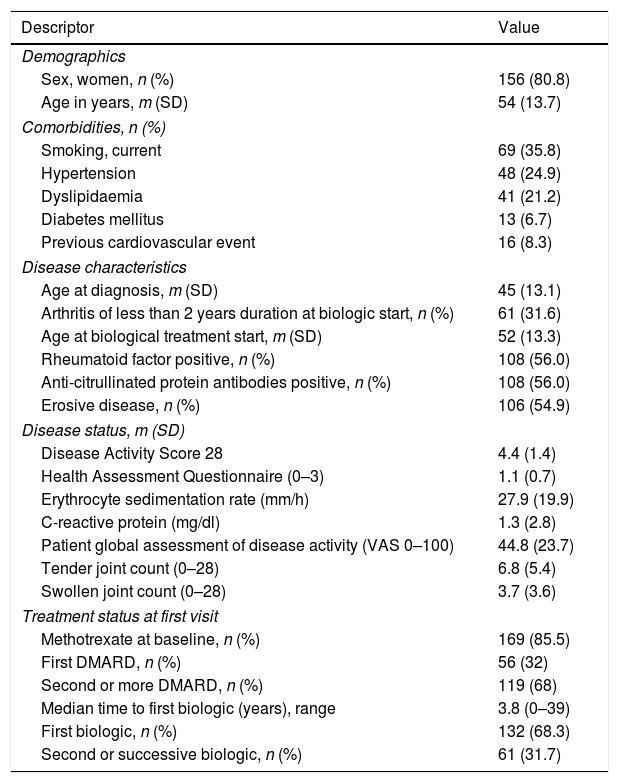
ResultsA total of 193 RA were started on a biological agent administered during the study period in the center. The majority of patients were women in their middle ages, and almost half had an erosive disease (see Table 1). As of first visit in the study more than 60% of patients had failed at least to two DMARDs and almost 32% had received other biological treatment; time to first biologic varied widely (see more details in Table 1). A third of the patients had an early arthritis (≤2 years).
Patients’ Characteristics at Baseline.
| Descriptor | Value |
|---|---|
| Demographics | |
| Sex, women, n (%) | 156 (80.8) |
| Age in years, m (SD) | 54 (13.7) |
| Comorbidities, n (%) | |
| Smoking, current | 69 (35.8) |
| Hypertension | 48 (24.9) |
| Dyslipidaemia | 41 (21.2) |
| Diabetes mellitus | 13 (6.7) |
| Previous cardiovascular event | 16 (8.3) |
| Disease characteristics | |
| Age at diagnosis, m (SD) | 45 (13.1) |
| Arthritis of less than 2 years duration at biologic start, n (%) | 61 (31.6) |
| Age at biological treatment start, m (SD) | 52 (13.3) |
| Rheumatoid factor positive, n (%) | 108 (56.0) |
| Anti-citrullinated protein antibodies positive, n (%) | 108 (56.0) |
| Erosive disease, n (%) | 106 (54.9) |
| Disease status, m (SD) | |
| Disease Activity Score 28 | 4.4 (1.4) |
| Health Assessment Questionnaire (0–3) | 1.1 (0.7) |
| Erythrocyte sedimentation rate (mm/h) | 27.9 (19.9) |
| C-reactive protein (mg/dl) | 1.3 (2.8) |
| Patient global assessment of disease activity (VAS 0–100) | 44.8 (23.7) |
| Tender joint count (0–28) | 6.8 (5.4) |
| Swollen joint count (0–28) | 3.7 (3.6) |
| Treatment status at first visit | |
| Methotrexate at baseline, n (%) | 169 (85.5) |
| First DMARD, n (%) | 56 (32) |
| Second or more DMARD, n (%) | 119 (68) |
| Median time to first biologic (years), range | 3.8 (0–39) |
| First biologic, n (%) | 132 (68.3) |
| Second or successive biologic, n (%) | 61 (31.7) |
Abbreviations: m, mean; SD, standard deviation; VAS, visual analog scale; DMARD, disease-modifying anti-rheumatic drug.
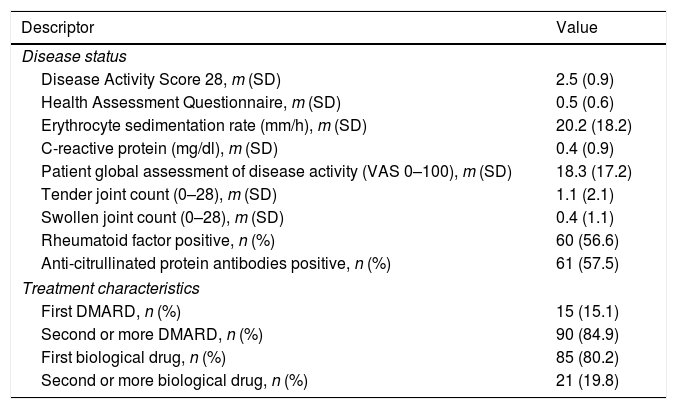
Mean follow-up in our series was 3.1 years (SD 2.1; range 0.5–10.4 years). Biological treatment was tapered in 106 (55%) of the patients and suspended in 34 (17.6%) at some point during follow-up. The biologic agent most frequently tapered was ETN (n=42; 39.6%), followed by ADA (n=39; 36.8%), IFX (n=15; 14.2%), TCZ (n=5; 4.7%), and RTX (n=5; 4.7%). No patient on abatacept (n=6) or golimumab (n=2) had their doses tapered. Mean time from biological treatment start to tapering was 5.3 years, with 50% of the patients being on such regimen before 4 years from initiation. Patients whose treatment was tapered or discontinued had a mean DAS28 of 2.5 (SD 0.9) and a mean HAQ of 0.5 (SD 0.6) at the visit when treatment was tapered or discontinued (see Table 2 for details on status).
Characteristics of the 106 Patients Whose Dose Was Tapered, at the Time of Dose-adjustment.
| Descriptor | Value |
|---|---|
| Disease status | |
| Disease Activity Score 28, m (SD) | 2.5 (0.9) |
| Health Assessment Questionnaire, m (SD) | 0.5 (0.6) |
| Erythrocyte sedimentation rate (mm/h), m (SD) | 20.2 (18.2) |
| C-reactive protein (mg/dl), m (SD) | 0.4 (0.9) |
| Patient global assessment of disease activity (VAS 0–100), m (SD) | 18.3 (17.2) |
| Tender joint count (0–28), m (SD) | 1.1 (2.1) |
| Swollen joint count (0–28), m (SD) | 0.4 (1.1) |
| Rheumatoid factor positive, n (%) | 60 (56.6) |
| Anti-citrullinated protein antibodies positive, n (%) | 61 (57.5) |
| Treatment characteristics | |
| First DMARD, n (%) | 15 (15.1) |
| Second or more DMARD, n (%) | 90 (84.9) |
| First biological drug, n (%) | 85 (80.2) |
| Second or more biological drug, n (%) | 21 (19.8) |
Abbreviations: m, mean; SD, standard deviation; VAS, visual analog scale; DMARD, disease-modifying anti-rheumatic drug.
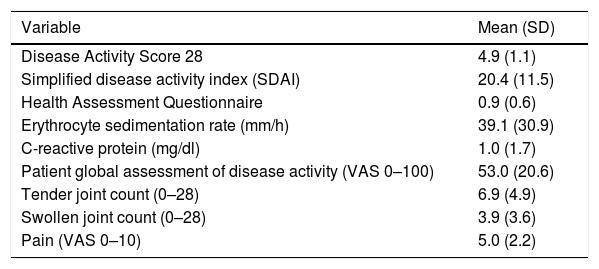
A relapse occurred in 38 patients (64.2%) during follow-up. The cumulative failure results were as follows: after 6 months, 10% of the tapered or discontinued treatments had ended on relapse, 19% at 12 months, 33.2% at 2 years, and 50% at 5 years. Mean time in tapered regimens was 4.6 years [95% confidence interval (95% CI): 3.7–5.4]. Clinical variables at relapse are displayed in Table 3. Patients experiencing a relapse returned to full standard doses, with reinstatement being effective to regain LDA in all patients except in 6 (15.7%).
Disease Status as of Time of Relapse.
| Variable | Mean (SD) |
|---|---|
| Disease Activity Score 28 | 4.9 (1.1) |
| Simplified disease activity index (SDAI) | 20.4 (11.5) |
| Health Assessment Questionnaire | 0.9 (0.6) |
| Erythrocyte sedimentation rate (mm/h) | 39.1 (30.9) |
| C-reactive protein (mg/dl) | 1.0 (1.7) |
| Patient global assessment of disease activity (VAS 0–100) | 53.0 (20.6) |
| Tender joint count (0–28) | 6.9 (4.9) |
| Swollen joint count (0–28) | 3.9 (3.6) |
| Pain (VAS 0–10) | 5.0 (2.2) |
Abbreviations: m, mean; SD, standard deviation; VAS, visual analog scale; DMARD, disease-modifying anti-rheumatic drug.
The odds ratios of potential predictors of relapse at the tapering visit were: DAS28 1.90 (95% CI: 1.32–2.74; P<.001); SDAI 1.11 (95% CI: 1.04–1.18; P=.001); HAQ 2.22 (95% CI: 1.41–3.52; P=.001); and VAS pain 1.24 (95% CI: 1.09–1.14; P=.001).
Only pain with an OR perincreasein0–10VAS=1.26 (95% CI: 1.11–1.43; P<.001) and ESR, with an OR perincreaseinmm/h=1.01 (95% CI: 1.00–1.03; P=.011) remained significantly associated with relapse at the multivariate model.
DiscussionWe have presented our experience with biological treatment tapering in RA patients. Our setting is a very homogeneous clinic with standardized procedures and we have shown that tapering and discontinuation in this setting is not only common practice but an effective solution that could be contemplated in patients with LDA or remission. In addition, we attempted to analyze potential risks factors for relapse after optimization and found that baseline ESR and the pain score where the only potential predictors.
In inflammatory arthritides biological treatment is commonly escalated, and, to a lesser extent tapered9 van Vollenhoven suggests that an important number of patients may receive unnecessary high doses of infliximab for many years.10 Our results reflect daily practice in our center, and have shown that some patients could benefit from the opposite, lower doses of biologics, while maintaining a good disease control for a long-time.
In 2014, a meta-analysis was conducted to assess the effectiveness of tapering compared to continuation of standard doses, concluding that dose reduction of ETN 50mg to 25mg weekly seems to be as effective as continuing the standard dose, after 3–12 months of LDA.11 The authors described some weaknesses, such as heterogeneity, the restriction to specific biological drugs, a short follow-up, and some methodological concerns. Due to these shortcomings, definitive recommendations could not be concluded.11 Some few years later, evidence from additional clinical trials has reached the quantities to allow another meta-analysis.12 Henaux et al. show that discontinuation increases twice the risk of losing remission, and increases 17% radiographic progression, compared to continuation, but tapering was not clearly related to losing low disease activity state.12 Our numbers are too low to separate discontinuation from tapering, but aggregated data from those treatment regimens are not very different from what is published from tapering versus continuation.
More than half of our patients had their biological treatment tapered at some point, and half of them were still in LDA or remission after 5 years of this scheme. In previous studies, other authors have shown similar figures.11,13,14 Tapering scheme was assigned according to common clinical practice, and in accordance to the recommendations for optimization from the Spanish Rheumatology Society and Hospital Pharmacy Society.15 Whether a specific prior length of biological treatment, or of time in remission or LDA, is required remains elusive. We could not find an association of relapse with neither prior time in LDA or with time to tapering, as some evidence points out16,17 although others who explored the issue previously could not find a strong association.11,14 Maneiro et al. found a higher relapse rate with abatacept than with other biologics, although this was evaluated in a small group of patients.14 Interestingly, we explored whether the drug was a factor that predicted relapse and it did not show statistical significance: the rate of relapse was similar across biologics. In addition, no abatacept treatment in our series was tapered.
Remission re-induction does seem an achievable target3,14 despite not being the case in a small proportion of our patients. It would be very interesting to know what factors predicted failure to therapy reinstatement; unfortunately, we could not address this question due to the small size of this group in our series.
We noted that biological treatment of some patients who met the criteria to be tapered remained at standard doses. This brings up the question of whether unmeasured factors preclude rheumatologists, or patients, from taking such a decision. The reasons for not tapering biological therapy have not been explored in depth, while the reasons to scale treatment have been.18 Fraenkel et al. in 2015 investigated how patients with RA approached risk-benefit trade-offs between remaining with their current treatment and adding a biologic.19 They found that impact of disease was both a reason to stay and to step-up treatment, and that subjects’ risk-benefit trade-offs were consistently modified by factors unrelated to medication, including sociodemographic characteristics, role responsibilities and the quality of the patient-physician relationship. In the case of tapering or discontinuation, we should definitely add factors influencing physician's attitude, which in turn could modify outcome, as part of the complex doctor-patient relationship.
This study presents several limitations and strengths. First, the small group of patients might affect the results. LDA could have correspond to spontaneous improvement or regression to the mean.20 It is also important to asses a possible radiological damage in the long run, giving that in a study were ETN dose was reduced, patients showed a slightly but clinically meaningful radiographic progression compared with participants who continued ETN.21 However, we did not evaluate comparatively treatments that were not tapered given the same level of patient’ disease activity. Other limitation could be the immunogenicity of biological drugs and that has not been taken into account in this work. Data on the use of agents blocking TNF, provide ample evidence of primary and secondary treatment inefficacy in patients with RA. Important issues relevant to primary and secondary failure of these agents in RA include immunogenicity, methodological problems for the detection of antidrug antibodies and trough drug levels, and implications for treatment strategies.22
To summarize, our study suggests that tapering could be considered in RA patients in LDA or remission with moderate to large guarantee to maintain LDA after 5 years; furthermore, low ESR and pain scores at baseline would increase the chances of staying free of activity. This observational single-center evidence could add to support sustainable treatment strategies in RA.
Ethical ApprovalThe protocol and materials of this study were approved by the Ethics Committee of Hospital Universitario Central de Asturias. Informed consent was waived due to the retrospective nature of the study. However, all patients who enter the unit sign a consent form by which they allow the staff to use their clinical data, adequately anonymized, for research purposes.
Data SharingData are available upon request to corresponding author.
FundingThis study did not receive specific funding.
Conflict of InterestAuthors declare no conflicts of interest with the results of the present study.
Professional medical writers in InMusc helped us present our study.