(1) To systematically and critically review the evidence of combined therapy with synthetic disease-modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis (RA) and (2) to design practical recommendations on their use.
MethodsA systematic literature review (SLR) was performed with a sensitive bibliographic search strategy in Medline, EMBASE and Cochrane Library. We selected randomised clinical trials that analysed the efficacy and/or safety of (1) combined therapy of synthetic compared with sequential therapy of synthetic DMARD in early RA and (2) combination of methotrexate+leflunomide or triple therapy with synthetic DMARD in established RA refractory to synthetic DMARD. Two reviewers made the first selection by title and abstract and 11 performed the selection after detailed review of the articles and data collection. The quality of the studies was evaluated with the Jadad scale. Based on the results, related recommendations were agreed upon in a nominal group meeting.
ResultsUltimately, no articles were included in the SLR. The analysis of the reviewed articles demonstrated the effectiveness of the treatment with synthetic DMARD following a “treat to target” strategy in early RA patients, and of combination therapy of synthetic DMARD in established RA refractory to synthetic DMARD. This resulted in 6 recommendations concerning combination therapy with synthetic DMARD.
ConclusionsThese recommendations aim to facilitate decision-making with the use of combined therapy with DMARD in RA.
1) Revisar sistemática y críticamente la evidencia sobre eficacia y seguridad de la terapia combinada con fármacos modificadores de la enfermedad (FAME) sintéticos en la artritis reumatoide (AR); 2) Emitir recomendaciones prácticas sobre su uso.
MétodosSe realizó una revisión sistemática de la literatura con una estrategia de búsqueda bibliográfica sensible en Medline, Embase y Cochrane Library. Se seleccionaron ensayos clínicos aleatorizados que analizasen la eficacia y/o seguridad de 1) la terapia combinada con FAME sintéticos comparada con la terapia secuencial con FAME sintético en la AR de inicio; y 2) la combinación metotrexato+leflunomida o la triple terapia de FAME sintéticos en la AR establecida refractaria a FAME sintéticos. Dos revisores realizaron la primera selección por título y abstract y 11 la selección tras lectura en detalle y la recogida de datos. La calidad se evaluó con la escala de Jadad. En una reunión de grupo nominal en base sus resultados se consensuaron una serie de recomendaciones.
ResultadosFinalmente no se incluyó ningún artículo en la RSL. Del análisis de los artículos revisados se encontró la eficacia en las AR de inicio del tratamiento precoz con FAME sintéticos siguiendo una estrategia «treat to target» y en AR establecidas refractarias a FAME sintéticos la de la terapia combinada con FAME sintéticos. Con ello se generaron 5 recomendaciones sobre la terapia combinada con FAME sintéticos.
ConclusionesEstas recomendaciones pretenden facilitar la toma de decisiones con el uso de la terapia combinada con FAME sintéticos en la AR.
The management of rheumatoid arthritis (RA) has changed enormously in recent years. New drugs and treatment strategies have completely changed the prognosis of these patients.1–4 Synthetic disease-modifying anti-rheumatic drugs (DMARDs) like methotrexate (MTX) or leflunomide (LEF) have been and continue to be essential therapies for the management of these patients.5–7 However, with new findings in the disease pathogenesis and the appearance of biologic therapies recommendations on their use has changed.8–10
For example, in the latest updating of the EULAR consensus document on RA management (2016),1 albeit with great controversy, one recommendation from the 2013 issue was finally excluded,11 which suggested in DMARD naive RA, regardless of the use of combined corticosteroids, conventional synthetic DMARD should be used as monotherapy or in combination therapy. There are different reasons for definitively excluding combined therapy in initial treatment, from the higher effect when combined with biologics compared to the combination of the DMARD themselves to the higher number of adverse events compared with monotherapy. In the same document and, regarding refractory patients or those with toxicity to initial treatment, if no bad prognostic factors present, therapy combined with MTX, LEF or salazopyrin (SSZ) is a recommended option and they show that the combination of the 3 is the most common therapy used.
However, even with this present, unresolved questions remain. For example, relating to the use of combined therapy with synthetic DMARD, although arguments against this are cited, are they really sufficient so as to exclude this model of treatment in early RA? Also, in the case of established RA which are refractory to initial treatment and where there are no poor prognostic factors, which combined therapy would be the most appropriate?
The NEXUS Project is an annual activity where, based on the best evidence and experience possible, an attempt is made to respond to issues in RA which are. As a result, and bearing in mind everything commented above, in the context of this project, the aim of this systematic literature review (SLR) was to evaluate some aspects on efficacy and safety of combined therapy with synthetic DMARD in RA and subsequently issue a series of practical recommendations to serve as guidelines for clinicians in their daily practice.
MethodsNEXUS ProjectThis publication forms part of the NEXUS Project. This is led by 2 national coordinators (rheumatologist experts) and comprises 8 work groups, each with a regional coordinator and 2 or 3 reviewers (depending on the group), for a total of 22 reviewers. Every year different subjects of interest in the field of RA are analysed. In the 2017–2018 issue this was the use of corticosteroids and combined therapy with synthetic DMARD in RA. In this publication we describe the SLR referring to the question on combined therapy in RA. The Spanish Society of Rheumatology guarantees that the methodology used is appropriate, but does not endorse the conclusions because the Spanish Society of Rheumatology has official policies in this regard.
Review protocolInitially, one of the national coordinators put forward the following questions which were responded to through an SLR:”In early RA patients, is combined therapy with synthetic DMARD better than sequential therapy with synthetic DMARD? In established RA patients who are refractory to standard first line therapy (synthetic DMARD), is the combination of MTX+LEF or triple synthetic DMARD therapy effective and safe?” The SLR protocol was defined with these questions.
PICO and study selection criteriaThe 2 clinical questions were transformed into the PICO with which the inclusion and exclusion criteria were defined. For the first questions we selected studies which included patients with RA (international criteria or clinical judgement), early RA (≤2 years onset) adults (≥18 years), DMARD naive; being treated with combined therapy (double or triple) with synthetic DMARD with or without corticosteroids or other adjuvant drugs. Comparative studies had to have synthetic DMARD in sequential therapy (monotherapies or DMARD therapy with add-on drugs).
For the second question we selected studies which included patients with RA (international criteria or clinical judgement), adults (≥18 years), established RA (> 2 years onset), refractory to standard first line treatment (synthetic DMARD); being treated with combined MTX and LEF therapy or triple therapy with synthetic DMARD, with or without corticosteroids or other adjuvant drugs.
In both questions articles were sought whose outcomes analysed efficacy and safety variables regularly used in the study of RA. Finally, only those studies with the following designs were included: met analysis, systematic reviews and randomised clinical trials (RCT). Studies on animals and basic science were excluded.
Search strategyAided by an expert documentalist search strategies were created for the different databases. For this they used the MeSH terms and terms in free text. Only articles on humans, in English or Spanish were included in the search.
For this review the following bibliographic data bases were screened: Medline, Embase and Cochrane Library (all from their initiation up until July 2017). Due to the volume of bibliographic references recovered, we decided not to review the grey literature of the main national and international rheumatology conferences. A manual search was subsequently performed secondary to the bibliography of the articles finally included. The supplementary material shows the search strategy used, together with the number of references collected.
All the references resulting from the searches were inserted into the EndNote programme for easier management.
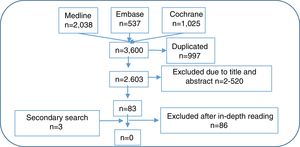
Article selectionFollowing this, 2 reviewers created the first selection of articles resulting from the search strategy by reading the title and abstract, complying with the inclusion and exclusion criteria, each independently. Whenever a discrepancy arose, a third reviewer was taken on board to make a decision. After this, 11 reviewers made a second article selection through independent detailed reading and applying the same inclusion and exclusion criteria. To do this, the number of references collected was equally distributed among the 11 reviewers. Whenever a discrepancy arose, the other reviewer of the previous phase resolved the problem. In Fig. 1 we show the flow diagram of the selection process of the articles, and in the supplementary material, the characteristics of the studies included and excluded.
Data collection and assessment of the study qualityThe 11 reviewers and one of the reviewers from the first selection stage, collected the study data included using specifically pre-designed templates). The Jadad12 scale was used to assess the methodological quality of the studies included. Again, where discrepancies arose the other reviewer from the previous stage resolved the problem.
Data analysis and presentationTables of evidence and outcomes were created, where the main characteristics and outcomes of the included studies were described. Some of these were expressed as numbers and percentages (%), mean and standard deviation, mean and interquartile range (p25–p75), others as odds ratios, relative risk or hazard ratios and their 95% confidence intervals (CI). The possibility of performing meta-analysis was only assessed where there was homogeneity.
Nominal group meeting and drawing up of recommendationsDuring a 2-day nominal group meeting which all NEXUS Project members attended, the outcomes of the SLR were presented and discussed. A series of recommendations were agreed to. Each of the recommendations, with guidance from the methodologist, was assigned a level of evidence and a level of recommendation, in keeping with the recommendations for evidence-based medicine from the Centre for Evidence-based Medicine in Oxford.13
ResultsOut of the 2603 references collected after the initial selection process 83 were assessed in depth and a further 3 through a secondary search. Finally, after an in-depth reading, no RCT were found which directly responded to the 2 questions (see excluded studies in the supplementary material). Below are comments on some of the studies excluded in this SLR which did not meet with the criteria to be included, but provide relevant data and ideas in relation to the 2 research questions. Table 1 outlines the main conclusions and recommendations.
Main conclusions and recommendations of the review.
| Conclusions | |
|---|---|
| In early onset RA… | |
| 1 | An early treatment with a treat-to-target strategy improves the activity, damage, function and quality of life parameters in the short and medium term and maintains them long term (LE 1b; GR B) |
| In patients with established RA refractory to synthetic DMARDs… | |
| 1 | The addition of LEF is better than MTX as monotherapy for improving activity, function and quality of life in the short to medium term, with no clear differences in AE (number of and severe AE) (LE 1b; GR B) |
| 2 | The combination of MTX+ETN appears to be superior to the combination of synthetic DMARD in optimising activity and function in the short term, particularly when the best outcomes are analysed (remission, ACR70) (LE 1b; GR B) |
| 3 | The superiority of MTX+LEF compared with MTX+HCQ o MTX+SSZ cannot be demonstrated in patients with established RA refractory to MTX, in the short term (LE 1b; GR B) |
| 4 | The combination of synthetic DMARD is similar to MTX+RTX (at doses of 500mg iv 2 doses) to improve activity and function in the short to medium term, with no clear differences relating to AE (number of and severe AE) (LE 1b; GR B) |
| 5 | Triple therapy with MTX+SSZ+HCQ improves parameters of activity and radiographic damage (LE 1b; GR B) |
| 6 | The efficacy of triple therapy with synthetic DMARDs is comparable with the combination of ETN+MTX (LE 1b; GR B) |
| 7 | The AE of triple therapy with MTX+SSZ+HCQ are those expected from the use of this type of drug (LE 1b; GR B) |
| Recommendations | |
| 1 | In patients with early RA a treat-to-target strategy is recommended, aimed at achieving remission as soon as possible (LE 1b; GA A) |
| 2 | In patients with established RA refractory to first line treatment with synthetic DMARD the option of using the combination of MTX y LEF is recommended, depending on the clinical context and the clinician's opinion (LE 1b; GR B) |
| 3 | If the combination of MTX+LEF (LE 5; GR D) is prescribed we recommend following the standard risk management guidelines. |
| 4 | In patients with established RA refractory to first line treatment with synthetic DMARD we recommend bearing in mind the option of using the triple therapy with MTX+HCQ+SSZ, depending on the clinical context and clinician's opinion (LE 1b; GR B) |
| 5 | Strict risk management is recommended in patients with RA who have been prescribed the triple therapy with MTX+HCQ+SSZ (LE 5; GR D) |
AE: adverse events; ACR: American College of Rheumatology; RA: rheumatoid arthritis; ETN: etanercept; DMARD: disease-modifying anti-rheumatic drugs; GR: grade of recommendation; HCQ: hydroxychloroquine; iv: intravenous; LEF: leflunomide; mg: milligram; MTX: methotrexate; LE: level of evidence; SSZ: salazopyrin.
The BeSt study was a study designed to analyse, the effect of different treatment strategies in early RA. Patients were assigned to one of the 4 groups and if there was no response a therapeutic decision was quickly taken.14–26 Patients therefore changed therapy (within their group) and even passed from one treatment branch to another. These groups were as follows: group 1 sequential monotherapy, group 2 combined sequential therapy (staged, i.e. monotherapy initiated and if response was insufficient another drug was added on), group 3 combined therapy with MTX+SSZ+prednisone (high doses with fast dose reduction) and group 4 combined therapy MTX+infliximab (IFX). Data of up to 10 years follow-up was available, where the RA activity was evaluated, in addition to function, structure damage, quality of life and safety.
In the BeSt study, after one year,17 the groups treated with MTX+SSZ+prednisone and MTX+IFX, demonstrated better control of the activity, function, radiographic damage progression than the other groups although they also presented with more adverse events (AE), with no differences being observed in bone mineral density. Progressively, in years 2–5,16,19,20,26 these differences between the groups practically disappeared and specifically after 5 years,20 48% of the patients were in clinical remission (DAS28<1.6) and 14% in drug-free remission, regardless of the initial treatment group. After 5 years it was published that radiographic damage was lower in those patients who had started treatment in one of the combination groups.20 After 10 years a subanalysis of ACPA-negative patients was performed reporting that the early use of MTX+SSZ+prednisone and MTX+IFX was more effective compared with MTX as monotherapy.
Question 2. In patients with established RA who are refractory to standard first line treatment (synthetic DMARD), is the combination of MTX+LEF or the triple therapy of synthetic DMARD effective and safe?Three good quality RCT27–29 were found (one is really data added onto the other 2 studies). They include almost 1000 patients with RA, mostly established RA (mean duration from 5 to 18 years) and refractory to standard synthetic DMARD, especially to MTX (Table 2). MTX was prescribed at a dose of 10–20 milligrams (mg)/week and LEF between 10 and 20mg/day. MTX in monotherapy was compared with MTX+etanercept (ETN), MTX+hydroxychloroquine (HCQ) and rituximab (RTX). All the patients included could take prednisone (up to 10mg/d). These RCTs analysed the efficacy (activity, function, quality of life) and safety of this combination up to 24 weeks.
Main characteristics and outcomes of the studies included with combined therapy of MTX and LEF.
| Study | Population | Intervention | Efficacy | Safety | |
|---|---|---|---|---|---|
| 1 | Fleischmann_2014 (aggregated data APPEAL+Latin RA), durat. 16 week., Jadad 4 | -Group ETN+MTX (n=478): 89% women, mean age 48 yrs., DAS28 mean 6.4, durat. RA 7 yrs.-Group HCQ+MTX (n=81): 86% women, mean age 49 yrs., DAS28 mean 6.6, durat. RA 8 yrs.-Group SSZ+MTX (n=95): 91% women, mean age 47 yrs., DAS28 mean 6.6, durat. RA 7 yrs.-Group LEF+MTX (n=69): 89% women, mean age 49 yrs., DAS28 mean 6.2, durat. RA 8 yrs. | -Group ETN+MTX-Group HCQ+MTX-Group SSZ+MTX-Group LEF+MTX | -% patients with ACR20Group ETN+MTX=82%Group HCQ+MTX=59% (P<.001 vs ETN+MTX)Group SSZ+MTX=54% (P<0.01 vs ETN+MTX)Group LEF+MTX=62% (P<.001 vs ETN+MTX)-% patients with ACR50Group ETN+MTX=56%Group HCQ+MTX=31% (P<.001 vs ETN+MTX)Group SSZ+MTX=20% (P<.001 vs ETN+MTX)Group LEF+MTX=38% (P<.05 vs ETN+MTX)-% patients with ACR70Group ETN+MTX=24%Group HCQ+MTX=12% (P<.01 vs ETN+MTX)Group SSZ+MTX=6% (P<.001 vs ETN+MTX)Group LEF+MTX=7% (P<.05 vs ETN+MTX)-% patients with low activity DAS28Group ETN+MTX=39%Group HCQ+MTX=20% (P<.01 vs ETN+MTX)Group SSZ+MTX=14% (P<.01 vs ETN+MTX)Group LEF+MTX=20% (P<.01 vs ETN+MTX)-% patients with remission DAS28Group ETN+MTX=18%Group HCQ+MTX=7% (P<.01 vs ETN+MTX)Group SSZ+MTX=4% (P<.001 vs ETN+MTX)Group LEF+MTX=9% (P not shown)-% patients with low activity CDAIGroup ETN+MTX=54%Group HCQ+MTX=30% (P<.01 vs ETN+MTX) (P<.05 vs SSZ+MTX)Group SSZ+MTX=18% (P<.001 vs ETN+MTX)Group LEF+MTX=42% (P<.001 vs SSZ+MTX)-% patients with remission CDAIGroup ETN+MTX=7%Group HCQ+MTX=2%Group SSZ+MTX=3%Group LEF+MTX=3% (P not shown)-Δ DAS28:Group ETN+MTX=−2.7Group HCQ+MTX=−2.0 (P<.001 vs ETN+MTX)Group SSZ+MTX=−1.5 (P<.001 vs ETN+MTX)Group LEF+MTX=−1.8 (P<.001 vs ETN+MTX)-Δ CDAI:Group ETN+MTX=−26.7Group HCQ+MTX=−21.1 (P<.001 vs ETN+MTX)Group SSZ+MTX=−17.4 (P<.001 vs ETN+MTX)Group LEF+MTX=−17.7 (P<.001 vs ETN+MTX)-Δ HAQ:Group ETN+MTX=−.77Group HCQ+MTX=−.47 (P<.001 vs ETN+MTX)Group SSZ+MTX=−.48 (P<.001 vs ETN+MTX)Group LEF+MTX=−.58 (P not shown) | -Not analysed |
| 2 | Kremer_2002, RCT double blind, active control, durat. 24 week., multicentre, international, Jadad 5 | -Group LFN+MTX (n=130): 76% women, mean age 55 yrs., durat. RA 10 yrs.-Group MTX+PBO (n=133): 76% women, mean age 55 yrs., durat. RA 10 yrs. | -MTX (15–20mg/week. or 10–15mg/week., if max. dose tolerated)+PCB-MTX+LFN 100mg/day, 2 days → LFN 10mg/day (if activity, ↑ dose to 20mg/day)-Prednisone 10mg/day | -% patients with ACR20MTX+LFN 46.2% vs MTX+PCB 19.5%, P<.001-% patients with ACR50MTX+LFN 26.2% vs MTX+PCB 6%, P<.001-% patients with ACR70MTX+LFN 10% vs MTX+PBO 2.3%, P=.015-Δ HAQMTX+LFN −.42 vs MTX+PCB −.09, difference=−.33 (95% CI −.44, −.21); P<.001-Δ SF-36 Physical componentMTX+LFN 6.8 vs MTX+PCB .3, difference=6.5 (95% CI 3.9, 8.7); P<.001-Δ SF-36 mental componentMTX+LFN=3.0 vs MTX+PCB 1.2 (ns) | -DiarrhoeaMTX+LFN=25.4%MTX+PCB=13.5%-Upper respira. tract infect.MTX+LFN=22.3%MTX+PCB=24.1%-NauseaMTX+LFN=16.2%MTX+PCB=11.3%-HeadacheMTX+LFN=10.0%MTX+PCB=8.3%-RashMTX+LFN=7.7%MTX+PCB=8.3%-DizzinessMTX+LFN=7.7%MTX+PCB=5.3%-InfectionsMTX+LFN=40.8%MTX+PCB=51.9%-ALT>3 ULNLFN+MTX 3.8%MTX+PCB .8%-AST>3 ULNLFN+MTX 1.5%MTX+PCB .8% |
| 3 | Wijesinghe_2017, RCT, double blind, active control, durat. 24 week., multicentre, national, Jadad 5 | -RTX+MTX (n=20): 80% women, mean age 44 yrs., DAS28 mean 6.88, durat. RA 5 yrs.-LFN+MTX (n=19): 95% women, mean age 48 yrs., DAS28 mean 6.43, durat. RA 18 yrs. | -RTX+MTX:RTX 500mg iv days 0 and 14 months+MTX-LEF+MTX: LFN 10mg/day op (up to 20mg/day)+MTX-All corticosteroids-NSAID and analgesics permitted | -RTX+MTX vs LFN+MTXACR20: 85% vs 84% (ns)ACR50: 60% vs 64% (ns)ACR70: 35% vs 32% (ns)No response ACR: 15% vs 16% (ns)DAS28: 3.26 vs 3.25 (ns)DAS remission (<2.6): 20% vs 26% (ns)DAS low activity (<3.2): 40% vs 42% (ns)DAS moderate activity (3.2–51): 60% vs 58% (ns)DAS high activity (>5.1): 0% vs 0%Moderate EULAR response 60% vs 58% (ns)Good EULAR response: 40% vs 42% (ns)NAD: 1.8 vs 1.16 (ns)CRP: 6 vs 3 (ns)ESR: 28.05 vs 30.42 (ns)RF: 84 vs 60 (ns)HAQ: 2.872 vs 2.132 (ns) | -RTX+MTXSevere AE n=5Infections n=4Unstable angina n=1Deaths n=0-LFN+MTXSevere AE n=3Infections n=1Cardiac events n=2Death n=1 |
Yrs.: years; AE: adverse events; ACR: American College of Rheumatology; RA: rheumatoid arthritis; CDAI: Clinical Disease Activity Index; DAS: Disease Activity Score; durat.: duration; RCT: randomised clinical trial; ETN: etanercept; RF: rheumatoid factor; HAQ: Health Assessment Questionnaire; HCQ: hydroxychloroquine; AMI: acute myocardial infarction; iv: intravenous; LEF: leflunomide; ULN: upper limit of normality; max.: maximum; mg: milligram; MTX: methotrexate; NAD: number of painful joints; ns: not significant; PBO: placebo; CRP: C-reactive protein; RTX: rituximab; week.: week; SF: Short Form; SSZ: salazopyrin; sup: superior; op: oral pathway; ESR: erythrocyte sedimentation rate.
The combination of MTX+LEF was statistically superior to MTX in monotherapy in ACR 20, 50 and 70, HAQ and SF-36 after 24 weeks of treatment in one of the RCT.28 However, another 16-week study found that, although all of the combinations analysed improved the activity and function of the patients, the combination of MTX+ETN was higher than MTX+LEF in many of the variables which evaluated the activity of RA (ACR 20, 50, 70, DAS28, CDAI) and the function. Furthermore, in this study it was not possible to show the superiority of MTX+LEF compared with MTX+HCQ or MTX+SSZ in these patients.27 In the third study included, the combination of MTX+LEF was similar to MTX+RTX (at intravenous doses of 500mg in 2 doses) to improve the activity (evaluated among others with the DAS28 and the EULAR response), in changes in the acute phase reactants and depending on the patients at 24 weeks.
Regarding safety, the expected AE were reported with the use of these DMARD, such as nausea, diarrhoea or elevated transaminases.27–29
With regards to efficacy and safety of the triple therapy in established and refractory RA, the SLR included 3 good quality RCT,30–33 which analysed the triple therapy with synthetic DMARD in patients with RA, most of which were established (Table 3). The duration of the RCT was from 1 to 2 years, and included over 400 patients with RA of 6–10 years onset, active (DAS28 mean of 6), refractory to synthetic DMARD. In all cases the triple therapy consisted of MTX at a dose of 20mg/week, SSZ 1–2g/day and HCQ 400mg/day. The comparators in these RCT were MTX as monotherapy, MTX+HCQ and ETN+MTX. All patients included could take stable corticosteroid doses (≤10mg/d) and triggers evaluated were the RA activity (clinical and analytical), overall patient assessment (OPA), structural damage and safety.
Main characteristics and outcomes of the studies included with triple therapy.
| Study | Population | Intervention | Efficacy | Safety | |
|---|---|---|---|---|---|
| 1 | O’Dell_1996, RCT, double blind, durat. 2 yrs., multicentre national, Jadad 5 | -MTX (n=36), 58% women, mean age 50 yrs., durat. mean RA 10 yrs., mean 1.6 previous DMARD-SZZ+HCQ (n=35), 72% women, mean age 49 yrs., durat. mean RA 6 yrs.-MTX+SSZ+HCQ (n=31), 65% women, mean age 50 yrs., durat. mean RA 10 yrs., mean 16 previous DMARD-CI: MTX 15–25mg/week. ≥12 week. Previous and DAS28 ≥4.4 | -MTX 7.5mg/week.-SSZ 500mg/12h+HCQ 400mg/day-MTX MTX 7.5mg/week.+SSZ 500mg/12h+HCQ 400mg/day-If no remission at 3 months ↑ MTX 12.5mg/week. → if no remission at 6 months ↑ MTX a 17.5mg/week.-Prednisone stable (≤10mg/d) and permitted NSAIDs | -ESRMTX=16 (ns vs triple therapy)SSZ+HCQ 16 (ns vs triple therapy)MTX+SSZ+HCQ 10-NPJMTX=7 (ns vs triple therapy)SSZ+HCQ=7 (P=.016 vs triple therapy)MTX+SSZ+HCQ=3-NSJMTX=5 (P=.006 vs triple therapy)SSZ+HCQ=7 (P=.001 vs triple therapy)MTX+SSZ+HCQ=2-MS (min)MTX=63 (ns vs triple therapy)SSZ+HCQ=50 (ns vs triple therapy)MTX+SSZ+HCQ=38-OPAMTX=3 (P=.020 vs triple therapy)SSZ+HCQ=3 (ns vs triple therapy)MTX+SSZ+HCQ=2-PGAMTX=2 (P=.002 vs triple therapy)SSZ+HCQ=3 (P<.001 vs triple therapy)MTX+SSZ+HCQ=1 | -no major differences between groups-MTX n=7 discontinuations (2 pneumonia, 1 stomatitis, 1 diarrhoea, 1 nauseas, 1 vertigo, 1 sepsis-death)-SZS+HCQ n=3 discontinuations (1 pneumonia, 1 diarrhoea,1 Crohn)-MTX+SSZ+HCQ n=3 discontinuations (1 nausea, 1 cervical cancer, 1 weight gain)-n=0 ↑ GOT ≥2 ULN |
| 2 | O’Dell_2013, RCT, no inferiority, double blind, durat. 48 week., multicentre national (RACAT study), Jadad 5 | -MTX+SSZ+HCQ (n=178), 77% women, mean age 58 yrs., durat. mean RA 5 yrs., DAS28 mean 5.8-ETN+MTX (n=175), 85% women, mean age 56 yrs., durat. mean RA 4 yrs., DAS28 mean 5.9 | -MTX (their regular dose)+SSZ 1g/day 6 week.→2g/day+HCQ 400mg/day-ETN 50mg/week.+MTX (their regular dose)-If DAS28↓<1.2 to 24 week. change to different regimen-SZZ could ↓ to 1g/day if AE-Prednisone stable (≤10mg/d) and permitted NSAIDs | -MTX+SSZ+HCQ vs ETN+MTX (48 week.)Δ DAS28 −2.12 vs −2.29 (ns)Δ HAQ −.46 vs −.64 (ns)Δ Modified Sharp score .54 vs 29 (ns)Δ CDAI −20.93 vs −21.56 (ns)DAS28 ≤3.2 37% vs 41.9% (ns)DAS28 ≤2.6 20.8% vs 25.2% (ns)ACR20 57.4% vs 65.8% (ns)ACR50 35.5% vs 42.6% (ns)ACR70 18.1% vs 26.5% (ns) | -MTX+SSZ+HCQ76.6% any AE11.3% severe AE29.7% gastroint disorder25.2% infections-ETN+MTX75.3% any AE11.9% severe AE21.5% gastroint disorder37.4% infections |
| 3 | Peper_2017, open extension of the RACAT study, durat. 72 weeks. | -MTX+SSZ+HCQ (n=145), 39% women, mean age 59 yrs., durat. mean RA 6 yrs., DAS28 mean 3.8-ETN+MTX (n=144), 47% women, mean age 56 yrs., durat. mean RA 5 yrs., DAS28 mean 3.5 | -MTX (their regular dose)+SSZ 1g/day 6 week.→2g/day+HCQ 400mg/day-ETN 50mg/week.+MTX (their regular dose)-Si DAS28 ↓ <1.2 to 24 weeks. Change to another regimen-SZZ could ↓ to 1g/day if AE-Prednisone stable (≤10mg/d) and permitted NSAIDs | -MTX+SSZ+HCQ vs ETN+MTXΔ DAS28 −3.03 (ns)NPJ, NSJ, OPA, ESR (ns)Adherence at one year 63% vs 78%Regimen changes greater from triple therapy to ETN+MTX (P=.005) | -Not assessed |
Yrs.: years; AE: adverse events; ACR: American College of Rheumatology; NSAID: non steroidal anti-inflammatory drugs; RA: rheumatoid arthritis; IC: inclusion criteria; CDAI: Clinical Disease Activity Index; Durat.: duration; DAS: Disease Activity Score; durat.: duration; DAS: Disease Activity Score; RCT: randomised clinical trial; ETN: etanercept; g: gram; gastroint: gastrointestinal; HAQ: Health Assessment Questionnaire; HCQ: hydroxychloroquine; ULN: upper limit of normality; mg: milligram; min: minutes, MTX: methotrexate; NAD: number of painful joints; NSJ; number of swollen joints; ns: not significant; week.: week; MS: morning stiffness; SSZ: salazopyrin; ESR: erythrocyte sedimentation rate.
In the first RCT,32 published in 1996, triple therapy was significantly superior to MTX as monotherapy for improving the number of swollen joints, the OPA score and the physician global assessment (PGA). Equally, the combination of SSZ+HCQ improved the number of swollen, painful joints and the PGA, from medium to long term (2 years). Notwithstanding there were no differences in the ESR and duration of morning stiffness. Neither were there any differences in terms of safety.
The RACAT study was a RCT of non inferiority, in which the triple therapy(MTX+SSZ+HCQ) was compared with the combination of MTX+ETN. After 24 weeks of treatment, MTX+ETN was statistically superior to the triple therapies in the percentage of patients who achieved low activity DAS28 (24.8% vs 34.8% P=.050), remission-DAS28 (12.7% vs 21.7% P=.030) and ACR70 (5% vs 16% P=.001), with no differences in the other variables, including the reduction mean of the DAS28 which was the mean of the main trigger. However, after 48 weeks (where the patients could be assigned to the other branch if response was insufficient), there were no statistically significant differences between groups relating to RA activity (DAS28, CDAI, ACR response 20, 50, 70), the HAQ and radiographic progression (Table 3). In open extension at 72 weeks,30 adherence to treatment at one year was higher in the ETN+MTX group than in the triple therapy group, 78% vs 63%, and changes of regimen were higher from triple therapy to ETN+MTX than the inverse (p=.005). The DAS28 continued to improve with no statistically significant differences between the groups.
Regarding safety, the expected AE were reported for the use of these DMARDs, including infections and gastrointestinal disorders.30–32
DiscussionIn this SLR we have tried to analyse some aspects relating to combined therapy with synthetic DMARD in RA patients. One of the questions relates to their use in early RA (this possibility is currently not included in the latest EULAR1 recommendations). The other question was raised to analyse the combination of MTX+LEF and the triple therapy of MTX+SSZ+HCQ, in established RA patient's refractory to standard treatment with synthetic DMARD.
Notwithstanding, after the selection processes of the SLR, no article was included which met all inclusion criteria and the questions raised could not be directly answered. Despite this, with analysis of the RCT excluded in the same, we could extract a series of conclusions and recommendations relating to the questions.
Firstly, relating to the use of the combined synthetic DMARD in early RA, we exposed the results of the BeSt study. After its ten years existence,14–22,24–26 we may conclude that what is really important is to begin early treatment and take decisions if the expected response is not obtained. Thus many studies in RA have demonstrated that an early diagnosis and treatment following a treat-to-target strategy clearly improves the prognosis of these patients.3,4,34 Although it is difficult to analyse, it has also been suggested that, although in the end the effect of the 4 groups is very similar, of the patients who began in some of the combined therapy groups, one included two synthetics DMARD: MTX+SSZ but also in combination with high dose glucocorticoids) and had a faster response (although including high dose corticosteroids in one group and biologic therapy in the other may have had a great impact on outcomes) and a lower radiographic progression (possibly conditioned by the previous point). Later, relating to the question for the patients with established RA refractory to synthetic DMARDs, the combination of MTX+LEF, and of MTX+SSZ+HCQ, as put forward by EULAR,1 may be therapeutic options to assess in this patient profile, despite the low or nil accumulated scientific evidence. Specific high quality studies are required in this regard.
Nevertheless, this SLR has several limitations. The first and most important was the difficulty in finding studies that fitted in with the PICO of the research questions, and the review inclusion criteria. This was so to such an extent that it was not possible to find any studies which could directly provide an answer to this. Also, even with the RCT which we have commented upon being available, some of them have very small sample sizes which limit outcome generality and others require more long-term data to conclude with greater assurance. For example, in the BeSt study, respondents were able to change treatment within their group change group (there were 4). In this study, similarly to others, the DMARD doses could be changed. All of this complicates statistics and its interpretation.
To conclude, although it has not been possible to answer all the questions raised directly in this SLR, we are convinced that the outcomes, conclusions and recommendations in this document may make a positive contribution to better knowledge of the use of synthetic DMARD in RA.
FinancingThe NEXUS Project was financed by Roche which did not participate in the choice of subjects, or development of review, conclusions or recommendations.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Roche for their involvement in the NEXUS Project. Also the members of the NEXUS group for their participation in review: Mercedes Alperi, Fred Antonio Anton Pages, Nagore Fernández-Llanio Comella. Also to Doctors Liliana Ercole and Estíbaliz Loza for their methodological and logistic coordination.
Please cite this article as: Calvo Alén J, Pérez T, Romero Yuste S, Ferraz-Amaro I, Alegre Sancho JJ, Pinto Tasende JA, et al. Eficacia y seguridad de la terapia combinada con fármacos modificadores de la enfermedad sintéticos en la artritis reumatoide: revisión sistemática de la literatura. Reumatol Clin. 2020;16:324–332.