Over the past decades, incidence of SLE (Systemic Lupus Erythematosus) has increased due to early case detection and improved survival of patients. SLE presents at an earlier age and has a more severe presentation in African-American, Native American, Asian, and Hispanic populations. Worldwide, lupus nephritis (LN) is observed in 29–60% of SLE patients, it has a negative impact in renal survival and patient mortality. Several cohorts have established potential risk factors associated with lupus nephritis, such as male sex, serological markers, and some extra-renal manifestations.
ObjectivesTo describe sociodemographic, clinical, immunological, and environmental risk factors in Colombian SLE patients and to compare the population with and without nephritis, in order to establish risk factors and possible associations.
Materials and methodsA total of 1175 SLE patients participated in this study. During medical care, an interview and structured survey was conducted and later registered in a database. Sociodemographic, clinical, immunological, and environmental exposure variables were analyzed. Bivariate and multivariate analyses were performed using presence of LN as an outcome.
ResultsPrevalence of LN was 38.7%. Variables significantly associated with LN included being male (OR 1.98), a duration of SLE>10 years (OR 1.48), positive anti-DNA (OR 1.34), positive anti-Sm (OR 1.45), and smoking (OR 1.66). Being non-smoker was a protective factor (OR 0.52).
ConclusionThis study describes potential factors associated with lupus nephritis in a Latin American population. Smoking status could be a target for intervention as it is a modifiable risk factor. The association between being male and LN is observed in Latin-American populations such as presented here. Further research in other large-scale population studies and more efforts are needed to gain better insights to explicate these relationships.
En las últimas décadas la incidencia del Lupus eritematoso sistémico (LES) se ha incrementado debido a la detección temprana y su mejoría en la supervivencia. La nefritis lúpica (NL) se observa en el 29% a 60% de los pacientes con LES, teniendo un impacto negativo en la supervivencia renal y la mortalidad. Varias cohortes han establecido factores de riesgo asociados con la NL, como el sexo masculino, marcadores serológicos y algunas manifestaciones extrarrenales.
ObjetivosDescribir los factores de riesgo sociodemográficos, clínicos, inmunológicos y ambientales en pacientes colombianos con LES y comparar la población con y sin NL para establecer posibles asociaciones.
Materiales y métodosSe incluyeron 1175 pacientes con LES, se analizaron variables sociodemográficas, clínicas, inmunológicas y ambientales, tomadas de bases de datos de registros cínicos. Los análisis bivariados y multivariados se realizaron utilizando la presencia de NL como desenlace.
ResultadosLa prevalencia de NL fue del 38,7%. Las variables significativamente asociadas con NL incluyeron sexo masculino (OR 1.98), una duración del LES>10 años (OR 1.48), anti-ADN positivo (OR 1.34), anti-Sm positivo (OR 1.45) y tabaquismo (OR 1.66), mientras que la ausencia de exposición al tabaco se comportó como factor protector (OR 0.52).
ConclusiónSe describen los factores potenciales asociados con NL en una población latinoamericana. El tabaquismo se presenta como un factor de riesgo susceptible de intervención. El sexo masculino y su asociación con NL ya ha sido reportado en otras poblaciones latinoamericanas. Se requieren investigaciones a gran escala en otras poblaciones para explicar mejor estas asociaciones.
Over the past decades, incidence of SLE (Systemic Lupus Erythematosus) has increased due to early case detection and improved survival of patients. SLE presents at an earlier age and has a more severe presentation in African-American, Native American, Asian, and Hispanic populations.1 Worldwide, lupus nephritis (LN) is observed in 29–60% of SLE patients,2 it has a negative impact in renal survival and patient mortality. Despite proper immunosuppressive therapy, up to 44% of lupus nephritis patients develop end-stage renal disease (ESRD), and 10-year overall survival ranges from 46% to 95%.2
Several cohorts have tried to address the risk factors associated with lupus nephritis. For example, the LUMINA cohort3 established that lupus nephritis occurs more frequently in Hispanics and African-Americans. Other studies have shown that variables such as male sex can be associated with a clinically different phenotype of the disease that has a more aggressive course and a higher incidence of renal involvement.4,5 Additionally, potential clinical predictors of renal involvement have been identified, finding a relationship of lupus nephritis with other clinical manifestations of SLE such as cardiovascular disease (i.e. arterial hypertension), serositis and cutaneous manifestations (i.e. malar rash). There is also a relationship with serological biomarkers (i.e. anti-Sm antibodies, low complement levels, high titers of antibodies against dsDNA), sociodemographic factors (i.e. age at onset, socioeconomic level), genetic and urinary markers.6 The identification of risk factors and predictors of poor prognosis may allow early interventions that could impact favorably on outcomes (Fig. 1).
Therefore, the aim of this study is to describe sociodemographic, clinical, immunological, and environmental risk factors in Colombian SLE patients, and compare the population with and without nephritis, in order to establish possible associations. With this study, the reader will be able to identify risk factors and predictors of poor prognosis associated with lupus nephritis in a population representative of Latin America ethnic groups.
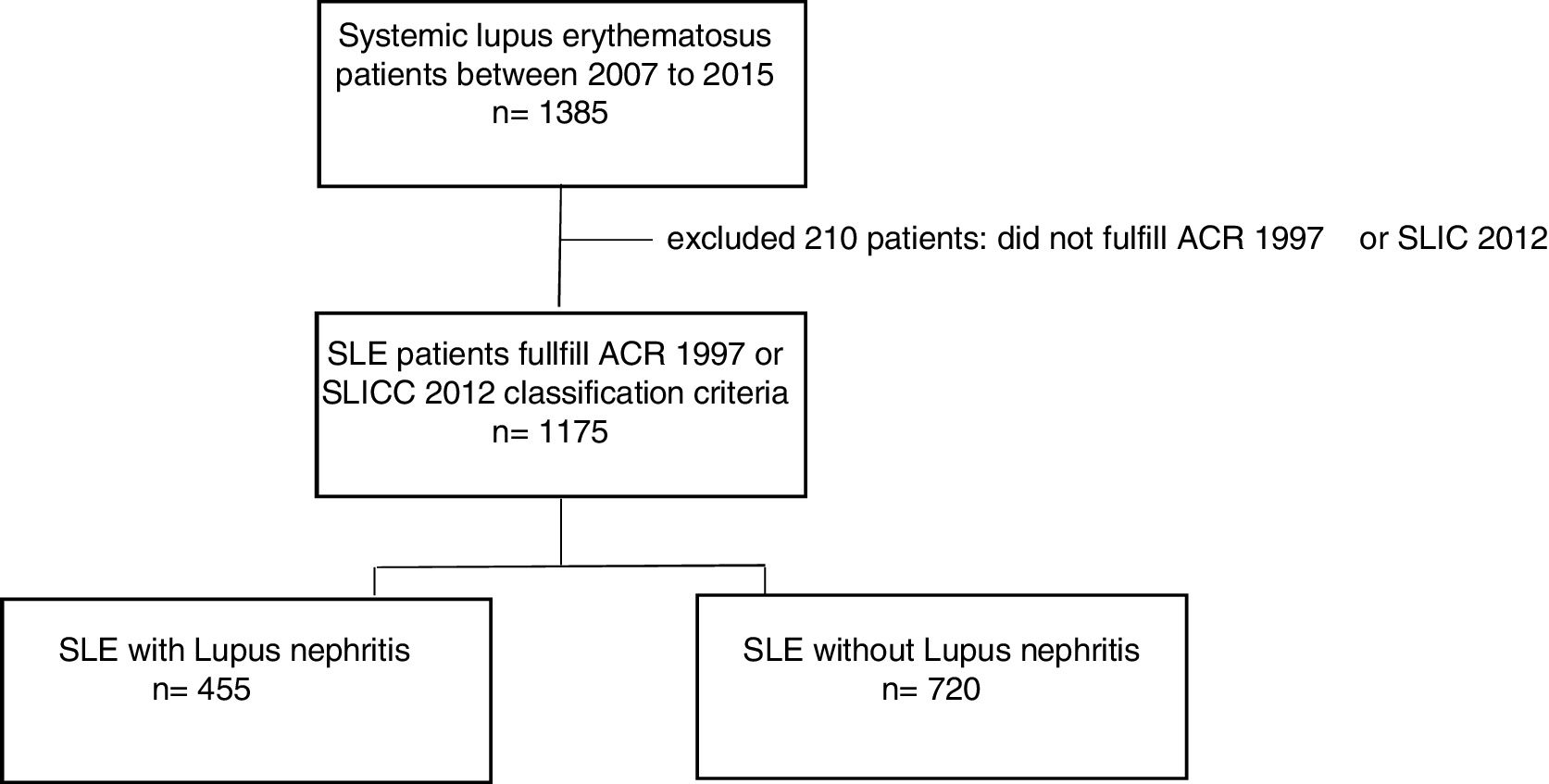
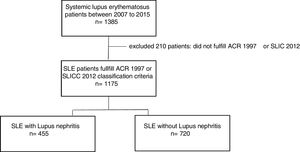
Materials and methodsStudy designThis is a cross-sectional study of SLE patients that were evaluated between 2007 and 2015 at an institution specialized in rheumatology. We excluded patients who did not meet the ACR 1997 or SLICC 2012 SLE classification criteria In addition, patients without enough clinical record information to label an individual as SLE patient by classification criteria were excluded. During medical care, an interview and structured survey was conducted and later registered in a database. Sociodemographic, clinical, immunological, and environmental exposure variables were analyzed.
PopulationParticipating patients resided in 7 different Colombian cities and were evaluated and treated at medical centers of Artmedica; which is the largest rheumatologic medical care center in Colombia. Artmedica evaluates the greatest number of individuals with autoimmune diseases nationwide.
EthicsThis study was carried out in accordance with the declaration of Helsinki and the Colombian law in resolution 8430 of 1993, article 11, which considers it a minimal risk investigation, given that retrospective documentary research techniques and methods were used. No intervention or intentional modification of the biological, physiological, psychological or social variables of the individuals who participated were carried out, nor were aspects of their behavior treated.
Sociodemographic variablesGender, age at study entry, duration of disease (since onset of SLE symptoms), age at diagnosis, schooling (elementary, high school, vocational/technical studies, college degree/professional), socioeconomic status were determined by the System of Identifying Potential Beneficiaries of Social Programs (SISBEN for its Spanish initials, 1–6 on a scale defined by the Colombian government authorities).
Clinical and immunological variablesThe presence or absence of the following characteristics were assessed: skin and mucosal manifestations (malar rash, discoid lupus, photosensitivity, oral ulcers), joint manifestations (arthritis), serositis (pericarditis, pleuritis), neurological manifestations (convulsions, psychosis), hematologic manifestations (leukopenia, lymphopenia, thrombocytopenia, and hemolytic anemia), and cardiovascular manifestations (coronary artery disease/myocardial infarction, hypertension, stroke, and upper and lower extremity thrombosis).
Renal involvement was confirmed with biopsy and histological classification (criteria of World Health Organization – WHO – or International Society of Nephrology/Renal Pathology Society 2003) and/or confirmed by the validated criteria of American College of Rheumatology (ACR); which considers persistent proteinuria as 0.5 grams/day or greater than 3+ on an isolated sample, and/or cell casts including erythrocytes, hemoglobin, granular, tubular, or mixed. Renal disease was staged from 1 to 5 following the international KDIGO guidelines for chronic kidney disease.
Polyautoimmunity, defined as the confirmed diagnosis of at least one additional autoimmune disease, was assessed in our patients. On the other hand, relevant immunological variables that were analyzed included: antinuclear antibodies (ANA), extractable nuclear antibodies (ENA, anti-Ro, anti-La, anti-SM, and anti-RNP), anti-DNA antibodies (anti-DNA), complement levels (C3 and C4), detectable levels of anti-cardiolipin IgG and IgM antibodies, and positive lupus anticoagulant antibodies.
Finally, the proportion of patients using specific treatments at study entry was established, which included steroids, anti-malarial drugs, conventional immunusuppressors, and biologic therapy.
Environmental exposure variablesIn our survey, data of the following exposures were obtained: smoking status (current or previous smoking or non-smoker), exposure to organic solvents and glues (including those commonly used in industry for pasting, degreasing, cleaning, laminating and flexing, painting and lubricating), hair dyes (at least once before or after being diagnosed with SLE), and use of prosthetic implants (permanent or removable).
Statistical analysisBivariate Chi-square analysis was performed for quantitative variables; Mann–Whitney U test was used for polytomous variables or for quantitative variables presenting non-normal distribution. Multivariate analysis by binary logistic regression (forward method) was performed considering presence of LN as an outcome. The model was adjusted for variables with statistically significant association with p<0.05 and variables with biological plausibility such as environmental factors and finally for possible confusing variables (i.e. socioeconomic status, duration of disease). Goodness of fit was assessed using the Hosmer–Lemeshow test.
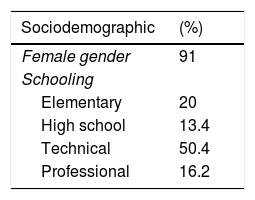
ResultsStudy populationOf the total 1175 SLE patients, 455 (38.7%) had lupus nephritis. Average age at diagnosis was 33 years and 91% were female. Among the clinical variables that stood out were hematologic, joint, and skin manifestations which were respectively found in 81.6%, 80.4% and 73.4%. Regarding environmental factors, current or previous smoking was reported by 20.7%, and use of hair dyes by 43.6%. Clinical, sociodemographic and autoimmune characteristics of our population study are shown in Table 1.
Characteristics of 1175 patients with systemic lupus erythematosus evaluated at a specialized center in Colombia between 2007 and 2015.
| Sociodemographic | (%) |
|---|---|
| Female gender | 91 |
| Schooling | |
| Elementary | 20 |
| High school | 13.4 |
| Technical | 50.4 |
| Professional | 16.2 |
| Use of exogenous agents | (%) |
|---|---|
| Smoking status | |
| Previous or current smoker | 20.7 |
| Non-smoker | 5.4 |
| Solvents | 2 |
| Hair dyes | 43.6 |
| Glue | 1.7 |
| Prosthetic implants | 1.8 |
| Clinical features | Mean (SD) |
|---|---|
| Age (years) | 44 (14) |
| Age at diagnosis (years) | 33 (13.5) |
| Duration of SLEa(years) | 10.6 (8.1) |
| Systemic manifestations | (%) |
|---|---|
| Skin and mucosa | 73.4 |
| Joints | 80.4 |
| Serosa | 20.7 |
| Neurologic | 5.4 |
| Hematologic | 81.6 |
| Renal | 38.7 |
| Autoimmune profile | (%) |
|---|---|
| Anti-DNA | 53.2 |
| Anti-SM | 31.6 |
| Low complement | 60.2 |
| Anti-cardiolipin IgG | 13 |
| Anti-cardiolipin IgM | 15.4 |
| Lupus anticoagulant antibody | 25 |
| Polyautoimmunity | 21 |
| Treatment | % |
|---|---|
| Steroid | 79 |
| Antimalarial | 69 |
| Azathioprine | 45 |
| Cyclophosphamide | 19.8 |
| Mycophenolate mofetil | 28.2 |
| Rituximab | 6.6 |
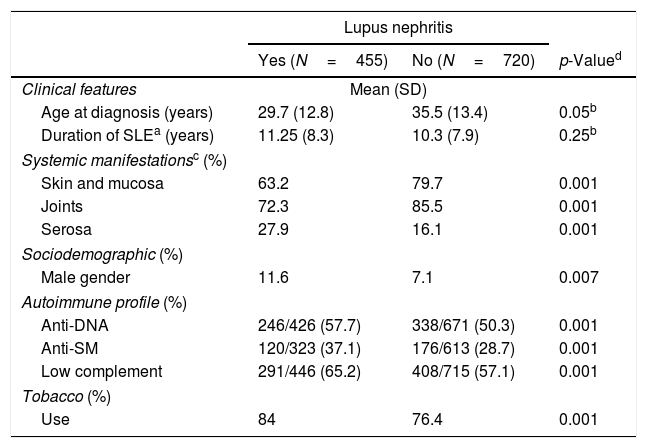
Lupus nephritis patients had an average age of 40.9 years and their average age at diagnosis was 29.7 years. Males were found to be more prevalent in the LN group. A longer time to disease progression was observed for LN patients, compared to patients without renal disease (11.25 vs. 10.3 years, respectively).
Regarding systemic manifestations, our data showed that LN patients exhibited significantly greater serous membrane involvement and fewer manifestations of the joints, skin, and mucosa. Positive serological markers were found in a significantly greater proportion of patients with LN compared to those without renal disease. For instance, LN patients were found to have low levels of complement, positive anti-DNA, and anti-SM in 65.2%, 57.7%, and 37.1% respectively. Characteristics of SLE patients according to renal disease status (LN vs. no renal disease) are shown in Table 2.
Clinical, immunologic, sociodemographic, and exposure in lupus nephritis patients.
| Lupus nephritis | |||
|---|---|---|---|
| Yes (N=455) | No (N=720) | p-Valued | |
| Clinical features | Mean (SD) | ||
| Age at diagnosis (years) | 29.7 (12.8) | 35.5 (13.4) | 0.05b |
| Duration of SLEa (years) | 11.25 (8.3) | 10.3 (7.9) | 0.25b |
| Systemic manifestationsc (%) | |||
| Skin and mucosa | 63.2 | 79.7 | 0.001 |
| Joints | 72.3 | 85.5 | 0.001 |
| Serosa | 27.9 | 16.1 | 0.001 |
| Sociodemographic (%) | |||
| Male gender | 11.6 | 7.1 | 0.007 |
| Autoimmune profile (%) | |||
| Anti-DNA | 246/426 (57.7) | 338/671 (50.3) | 0.001 |
| Anti-SM | 120/323 (37.1) | 176/613 (28.7) | 0.001 |
| Low complement | 291/446 (65.2) | 408/715 (57.1) | 0.001 |
| Tobacco (%) | |||
| Use | 84 | 76.4 | 0.001 |
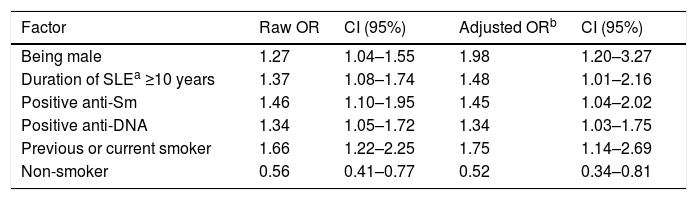
Compared to females, male patients exhibited a 1.98 times higher risk of presenting lupus nephritis. Patients with disease duration longer than 10 years had a 1.48-fold increased risk of LN. Similarly, positive anti-DNA (OR 1.34 CI95% 1.03–1.75) and positive anti-SM (OR 1.45 CI95% 1.04–2.02) were associated with the presence of lupus nephritis.
Regarding exposure to environmental factors, previous or current history of smoking increased 1.75-fold the risk of LN, while having a non-smoking status was protective (Table 3).
Factors associated with lupus nephritis in a Colombian SLE patients.
| Factor | Raw OR | CI (95%) | Adjusted ORb | CI (95%) |
|---|---|---|---|---|
| Being male | 1.27 | 1.04–1.55 | 1.98 | 1.20–3.27 |
| Duration of SLEa ≥10 years | 1.37 | 1.08–1.74 | 1.48 | 1.01–2.16 |
| Positive anti-Sm | 1.46 | 1.10–1.95 | 1.45 | 1.04–2.02 |
| Positive anti-DNA | 1.34 | 1.05–1.72 | 1.34 | 1.03–1.75 |
| Previous or current smoker | 1.66 | 1.22–2.25 | 1.75 | 1.14–2.69 |
| Non-smoker | 0.56 | 0.41–0.77 | 0.52 | 0.34–0.81 |
Binary logistic regression.
a. Variables specified on step 1: Presence of polyautoimmunity, use of chemicals, use of solvents, use of glue, use of hair dyes, time of SLE progression (years), socioeconomic status, years of schooling, smoking status, positive anti-DNA, positive anti-Sm, gender, age, non-smoking status.
Throughout the literature, the Latin American population is considered a single minority group, despite it being a heterogeneous population with differences in their ancestry, socioeconomic, and cultural conditions. This diversity may play a role in favoring manifestations of SLE such as worse outcomes and differences in clinical response, as was shown by the Latin-American Lupus Study Group (GLADEL, Grupo Latinoamericano de Estudio del Lupus), which is the largest study of Latin American SLE patients.7
Our population study included 1175 SLE patients, being one of the largest compared to regional and worldwide studies. In this population, prevalence of lupus nephritis, distribution by gender, average age at diagnosis, and time course of disease progression in patients with or without LN were similar to those previously reported by other Colombian, Latin-American, and North American studies.6–10 Our data showed that patients with LN presented fewer joint, skin and mucosa manifestations than those reported by a previous Colombian study6; that reported that pleuritis was significantly associated with lupus nephritis, suggesting a SLE sub-phenotype in which these two manifestations are associated. In our study however, this association was no longer observed after adjusting for different clinical and paraclinical variables.
While ethnic distribution was not analyzed in our study, the previous GLADEL study reported that African-Latin American (ALA) patients were more prevalent in Colombia than Caucasians, Mestizo (mixed European and Amerindian ancestry), or pure Amerindians.11 Subsequent analyses of that same cohort have shown that ALA, as well as Mestizo patients, exhibited higher risk of developing renal disease, which was presented earlier and was more severe.7 Similarly, results from the LUMINA (Lupus In MInorities: NAture versus nurture) study showed that Hispanics and African-Americans were at increased risk of lupus nephritis.3 Thus, the role of ethnicity as a predictor of risk of LN may be explained by the variation in distribution of ancestral alleles involved in SLE and LN physiopathology.12 Therefore, the Latin America population represents an ethnic group at high risk of SLE/LN and thus identifying associated factors may ultimately have prognostic implications.
Several environmental exposure factors have been implicated in the development of SLE in previously healthy individuals, in SLE physiopathology, and in the development of more aggressive forms of the disease. Among these factors, exposure to tobacco has been widely studied and it has been proposed that it may trigger epigenetic modifications – similar to that observed in other autoimmune diseases such as rheumatoid arthritis – that lead to increased oxidative stress, systemic inflammation, upregulation of inflammatory cytokines, and production of autoantibodies.13 In agreement with this, the Nurses’ Health Study, in which a large cohort of patients in the United States were followed for decades, found an association between smoking and >10 pack-years of smoking with SLE and positive anti-dsDNA antibodies.14 In addition, smoking during pregnancy, as well as exposure of newborns to secondhand smoke, increased risk of childhood onset SLE.15 Among the evaluated environmental factors in our study, current or previous history of smoking was associated with increased risk of developing LN. Freemer et al. showed that active smoking was associated with positive anti-dsDNA, and in turn, this positivity was associated with LN; however, a direct association between smoking and LN was not reported.16
In our patients with LN, the prevalence of ESRD (stage 5 CKD) was 7%, in contrast to the prevalence of 26% in the cohort reported by Ward et.al. in which a 2.5-fold increase in CKD disease progression was observed in the smoking LN population compared to the non-smoking LN population.17 Unfortunately, limitations in our epidemiological design do not allow this comparison. Likewise, that study showed that cigarette smoking and hypertension negatively impact the time to development of ESRD in LN patients. A theory proposed to explain this observation is that the use of tobacco leads to increased levels of thromboxane A2, which may boost renal vasoconstriction and platelet aggregation, precipitating chronic vascular changes of lupus nephritis. However, our results and that of others, were not able to demonstrate this association.18 Importantly, our data showed that a non-smoking status constitutes a protective factor for LN, which had not been previously reported as statistically significant.
Tobacco use has been implicated as one of the risk factors that, together with non-traditional risk factors inherent to SLE inflammation, favors development of cardiovascular disease.19 Furthermore, an association between smoking and nephritis with metabolic syndrome has been previously described in Latin American SLE patients.20 Similarly, previous history of nephritis as well as of tobacco use have been associated to thrombosis in a multi-ethnic cohort involving Hispanic SLE patients.21 In conclusion, tobacco exposure constitutes a relevant variable to take into account not only as a risk factor for SLE, CVD, or thrombosis, but also as a risk factor for development of lupus nephritis.
Results from our analysis showed that male SLE patients were at increased risk of LN, which is in agreement with previous studies. While SLE most frequently affects the female population, males present a clinically distinct phenotype, which is more aggressive and has a higher incidence of renal disease.4
This association has been addressed by studies in other regions worldwide. In Latin America, the GLADEL cohort found a significantly higher prevalence of LN in males compared to females (58.5% vs 44.6% p=0.004).22 Molina and colleagues obtained similar results (58% vs 44% p=0.004), and identified a non-statistically significant trend of increased frequency of dialysis and renal transplant in male LN patients.23 In the LUMINA population study, in which Hispanics could represent our population, a greater prevalence of LN was identified in male patients, although not being statistically significant (63.5% vs 52.1% p=0.085).24
In a cohort of Latin American lupus nephritis patients, the presentation of nephrotic syndrome and type IV LN were more frequent in males compared to females, while no differences in progression or remission of LN were identified among genders.25 Similar findings have been described in an African-American population in the United States,26 and several studies in Europe,27 including the Spanish RELESSER cohort, in which 353 male patients were compared to 3298 female patients, and a greater prevalence of LN was also observed in males (44.8% vs. 29.8% p>0.001).28 In contrast, the study by Mok et al.29 in a Chinese population did not find differences in prevalence of LN among genders, supporting the relevant role of ethnicity in physiopathology and target organ damage in SLE.
Traditionally, estrogens have been considered a risk factor for SLE and being male as a protective factor for SLE incidence. Given the current evidence of a clinically aggressive phenotype of SLE in males, several studies have attempted to propose a biologic explanation for this observation. Hughes and colleagues4 genotyped over 6000 patients including female and male SLE cases and controls, and found that male patients presented a higher frequency of risk alleles, which may explain the severity of their disease. Regarding the increased frequency of renal disease observed in male SLE patients, other studies of CKD have shown that 17B-estradiol is able to inhibit pro-inflammatory and pro-apoptotic events exerting a nephroprotective role, while androgens stimulate programmed cell death via Fas/FasL. Additional hypotheses involving translocations and Toll-like receptors (TLRs) have also been proposed.4
In conclusion, we have described and analyzed the largest sample of Colombian lupus nephritis patients in which we have provided strong evidence to show that our population is demographically, clinically, and immunologically comparable to those from other geographic areas.
The present study has some limitations, mainly those related to the cross-sectional design which is observational. As the name advises, the aim of a cross sectional study is to obtain a representative sample by taking a cross section of the population as we do in the present work. All the measurements for the participants of the study were obtained at a single point in time. Although cross sectional studies are useful for assessing the disease burden in a descriptive way and for hypothesis generation, they are limited in confirming causality and in establishing temporal associations between exposure and the disease or condition (in the present case, lupus nephritis). In fact, they are prone to selection and information bias and confounding. Therefore, caution is needed in interpreting the present results.30
Even though more high-quality research is needed, in agreement with previous studies, our results show that current smoking is a risk factor for LN, while non-smoking is a protective factor. Importantly, this constitutes a target for intervention, as it is a modifiable risk factor. Previous studies in Latin American and Hispanic populations have reported the association between being male and LN, while additional studies in other continents and ethnicities have been heterogeneous, thus supporting the possible role of ethnicity, ancestry and racial admixture in SLE physiopathology. In order to gain better insights to explicate these relationships, more efforts and further research in other large-scale population studies are needed. The association between being male and LN is observed in Latin-American populations, while not in other ethnicities. Thus, ancestry and racial admixture may play a role in SLE physiopathology.
Conflict of interestsThe authors declare that there are no conflicts of interest.