In systemic sclerosis (SSc), peripheral vasculopathy presents typically as Raynaud Phenomenon (RP) and Digital Ulceration (DU). Over the last decade, botulinum toxin (BT) has been reported effective in this scenario. Our goal was to review existing literature evaluating the efficacy of BT on RP/DU in SSc.
Materials and methodsWe performed a search in Pubmed with the MeSH terms “systemic sclerosis” and “botulinum toxin”. Original studies evaluating BT in the treatment of SSc-associated RP/DU were considered for inclusion. Results were screened by title, abstract and full-text.
ResultsWe identified 30 results, of which 5 original papers were included: 2 randomized controlled trials (RCT), 2 case series and 1 case control study, from a total of 133 patients. Only one RCT showed negative results, with worse blood flow in treated arm, but with lower dose of BT. Despite this, all 5 included studies reported improvement of at least 1 RP/hand function outcome measure. Concerning DU healing, resolution of baseline DU at the end of follow-up was reported in 75–100% of the patients, with 1 RCT showing superiority over placebo. The only reported adverse effect was transient hand weakness, affecting only 0–16.7% of patients. BT injection protocols were highly heterogeneous.
ConclusionDespite conflicting results in 1 RCT, evidence points BT as an option in the treatment of SSc-related peripheral vasculopathy. However, future larger prospective trials are necessary to corroborate this hypothesis.
En la esclerosis sistémica (ES), la vasculopatía periférica se presenta normalmente como fenómeno de Raynaud (FR) y ulceración digital (UD). En el último decenio se ha reportado la efectividad de la toxina botulínica (TB) en este escenario. Nuestro objetivo fue revisar la literatura existente que evalúa la eficacia de la TB en el FR/UD en la ES.
Materiales y métodosRealizamos una búsqueda en Pubmed con los términos MeSH «esclerosis sistémica» y «toxina botulínica». Se consideraron para inclusión los estudios originales que evaluaban la TB en el tratamiento del FR/UD asociados a ES. Se cribaron los resultados por título, resumen y texto completo.
ResultadosIdentificamos 30 resultados, de los cuales se incluyeron 5 documentos originales: 2 ensayos controlados aleatorizados (ECA), 2 series de casos y un estudio de control de caso, de un total de 133 pacientes. Únicamente un ECA reflejó resultados negativos con peor flujo sanguíneo en el brazo tratado, aunque con menor dosis de TB. A pesar de ello, los 5 estudios incluidos reportaron una mejora de al menos una medida del resultado FR/función de la mano. En cuanto a la sanación de la UD, la resolución de la UD basal al final del seguimiento se reportó en el 75-100% de los pacientes, y un ECA reflejó superioridad con respecto al placebo. El único efecto adverso reportado fue debilidad transitoria en la mano, que afectó únicamente al 0-16,7% de los pacientes. Los protocolos de inyección de la TB fueron altamente homogéneos.
ConclusiónA pesar de los resultados conflictivos en un ECA, la evidencia apunta a la TB como opción para el tratamiento de la vasculopatía periférica asociada a la ES. Sin embargo, son necesarios ensayos prospectivos futuros más amplios para corroborar esta hipótesis.
Systemic sclerosis (SSc) is an orphan connective tissue disease where diffuse microangiopathy and immune system dysregulation result in collagen hyperproduction with skin and internal organs fibrosis.1 Raynaud Phenomenon (RP) is a consequence of peripheral microvasculopathy, triggered by endothelium dysfunction.1,2 It is highly prevalent in SSc (95% of patients) and consists on an episodic colour change of the extremities in response to cold exposure.3 Moreover, it is typically the initial manifestation and precedes by years major organ involvement.1 Digital ulcers (DU) are a serious consequence of SSc related vasculopathy. They occur in up to 58% of patients, either in the diffuse or limited subtype. With an extended time to healing, DU may result in critical ischaemia and soft tissue/bone infections, thus demanding aggressive treatment. Moreover, they also point to a worse prognosis.4
As vascular injury performs a major role in SSc pathogenesis, several treatment options focuses on it, not only for RP and DU, but also pulmonary arterial hypertension. Nowadays, calcium channel blockers, prostacyclin analogues, endothelin receptor antagonists and phosphodiesterase inhibitors are the main pharmacologic representatives to target this pathway.5 Nevertheless, in daily clinical practice, RP and DU still pose a challenge for both physicians and patients.
In the last two decades, botulinum toxin (BT) has emerged as a nonsurgical treatment for vasospastic disease.6Through local hand injections, numerous reports showed an improvement in RP severity and DU healing,6,7 including in patients with SSc.
The aim of this review was to evaluate the available evidence concerning the use of BT in the treatment of SSc related RP/DU.
Materials and methodsData source and search strategyA literature review was devised, fitting the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines in order to identify all full-text manuscripts that focused on the use of BT in the treatment of SSc related RP/DU. The search was performed on Pubmed, with the following MeSH terms: ‘systemic sclerosis’ and ‘botulinum toxin’, with the boolean term “AND”. No other keywords were added, in order to avoid an excessively restrictive search string that would exclude studies of interest and result in an extremely low number of results.
The search was performed from database inception up to the 31st of May 2019. No filters were added.
Screening process and selection criteriaAfter the search, MG and DF screened the records on 3 steps – title, abstract and full-text level – to assess for inclusion. A manual search through the references of the retained manuscripts was also performed in order to detect additional reports. Records were considered eligible when both reviewers included them for the next step. When opinions differed, consensus was reached by discussion with the remaining investigators (BS, TV, PP).
Manuscripts were selected considering the Population, Intervention, Comparison, Outcome (PICO) strategy:
- -
Population: Patients aged 18 or more years old, with SSc related RP or DU refractory to standard of care. Ideally, studies should follow the 2013 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) and/or 1980 ACR classification criteria. Cohorts with broader samples that included SSc patients were also considered for inclusion;
- -
Intervention: BT hand injection;
- -
Comparison: Ideally, randomized control group of patients maintaining standard of care. In the absence of a control group, a statistical analysis comparing with baseline was considered the minimum;
- -
Outcome: for DU, ulcer healing and development of new ulcers. For RP, Raynaud's Condition Score (RCS) or, in alternative, at least 1 Visual Analogue Scale (VAS). Occurrence and description of complications.
Reviews, letters, editorials, abstracts of scientific reunions and case series (CS) with less than 5 patients were rejected. English, French and Portuguese were the only 3 idioms accepted. After screening, manuscripts’ data was systematized in a standardized electronic spreadsheet that included: author, year, country, study design, SSc classification criteria, sample size, type of BT used, injection protocol used (including injection points and dose administered), evaluated outcomes, follow-up length, adverse reactions, statistical analysis performed and results.
Quality appraisalThe studies selected through the screening process were assessed for quality appraisal by MG and DF using the National Institute of Health (NIH) tools for randomized control trials (RCT), case–control (CC) studies and CS.
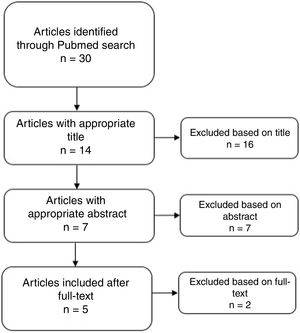
ResultsThirty results were obtained from the search through Pubmed. No article was obtained by reference checking. After the screening phase, 5 reports were considered for the qualitative analysis (see Fig. 1). Ratings according to the NIH are presented in supplement 1. Only 1 was considered as Good Quality.8
Study design, population and exclusion criteriaTwo of the retained articles were RCT and presented the largest samples, 408 and 459 patients (Table 1). Both used the American College of Rheumatology (ACR) 1980 and/or ACR/European League Against Rheumatism (EULAR) 2013 classification criteria. Bello et al.8 applied the most rigorous exclusion criteria. In comparison to Motegi et al., 2017,9 the first authors also selected a more suitable control group – the contralateral hand of each patient, in a double-blinded design.
Demographic characteristics of studies included in the systematic review.
| Study | Country | Study design | Classification criteria | Exclusion criteria | Sample size | Control group |
|---|---|---|---|---|---|---|
| Bello et al., 20178 | United States of America | Double blinded RCT | ACR 1980 or ACR/EULAR 2013 | Active infection, acute digit ischaemia, myasthenia gravis, hypersensitivity to BT, previous BT vaccine, current use of aminoglycoside antibiotics, prior upper extremity vascular surgery, pregnancy, lactation | 40 | Contralateral hand |
| Motegi et al., 201611 | Japan | CS | ACR 1980 or ACR/EULAR 2013 | Previous treatment with BT, pregnancy | 10 | Not applicable |
| Motegi et al., 20179 | Japan | Single blinded RCT | ACR 1980 or ACR/EULAR 2013 | Previous treatment with BT, pregnancy | 45 | 8 randomly selected patients |
| Serri et al., 201312 | France | CS | Not reported | Hypersensitivity to BT, pregnancy, lactation, radial/ulnar artery occlusion | 18 | Not applicable |
| Uppal et al., 201410 | United Kingdom | CC | Not reported | Not reported | 20 | Dominant hand |
ACR, American College of Rheumatology; BT, Botulinum toxin; CC, Case control; CS, Case series; EULAR, European League Against Rheumatism; RCT, Randomized controlled trial.
The CC study10 failed to report on classification and exclusion criteria. Despite this, it followed a methodology similar to the one adopted by Bello et al.8 concerning the control group, by using the patient's dominant hand, but with no blinding process.
The 2 CS presented the smallest samples (Motegi et al., 201611 with 10 patients, Serri et al.12 with 18 patients), thus under-powering even more their conclusions. One of them also didn’t report on classification criteria.12
As for current treatment, there was disparity between studies. All authors implemented BT as adjuvant treatment. In 3 of them, patients were included only if there were unsatisfying results with standard of care.9–11 Serri et al.12 and Bello et al.,8 on the contrary, included patients irrespective of treatment response. None of the 5 described clearly the previous treatment, specifically which associations of vasodilators were used. The same was verified concerning extra-articular manifestations.
Outcome measuresTable 2 summarizes the evaluated outcome measures. RCS is a validated tool to assess the severity of RP; however, it was only applied in 3 studies.8,9,11 Other tools used to evaluate this outcome were: VAS of pain (Pain-VAS), skin temperature recovery time, McCabe cold sensitivity score, re-colouration time, colour change VAS and cold intolerance VAS.
Outcome measures of studies included in the systematic review.
| Study | Raynaud's phenomenon | Digital ulcers | Hand function | Perfusion | Follow-up visit |
|---|---|---|---|---|---|
| Bello et al., 20178 | RCS, Pain-VAS, McCabe cold sensitivity score | Number of DU | QuickDASH | Change in blood flow measured with Laser Doppler Imaging; pulse oximetry | Week 4 & 16 |
| Motegi et al., 201611 | RCS, Pain-VAS, skin temperature recovery time after cold water stimulus | Number of DU | Not evaluated | Not evaluated | Week 2, 4, 8, 12 & 16 |
| Motegi et al., 20179 | RCS, Pain/numbness-VAS, skin temperature recovery time after cold water stimulus | Number of DU | Not evaluated | Not evaluated | Week 4, 8, 12 & 16 |
| Serri et al., 201312 | Pain VAS, patient satisfaction scale, re-colouration time | Number of DU | QuickDASH | Oxygen partial pressure | Week 4 |
| Uppal et al., 201410 | Pain VAS, colour change VAS, cold intolerance VAS | Presence of DU | Pinch/power grip strength, range of movement of hand joints, hand span, Kapandji thumb opposition test, DASH | Not evaluated | Week 8 |
DASH, disability of arm, shoulder and hand questionnaire; QuickDASH, Quick Disabilities of the Arm, Shoulder and Hand questionnaire; DU, digital ulcer; RCS, Raynaud's condition score; VAS, visual analogue scale.
Laser Doppler imaging and Oxygen Saturation were performed by Bello et al.8 to objectively evaluate perfusion. Serri et al.,12 on the other hand, only used Oxygen Partial Pressure. All included manuscripts evaluated the presence/number of DU.
As for hand function, a high variety of measures were applied. Motegi et al.9,11 was the only that did not include hand function evaluation. The “Disabilities of the Arm, Shoulder and Hand” questionnaire (DASH) is a validated tool that evaluates upper limbs daily functionality, and was used by Uppal et al.10 Bello et al.8 and Serri et al.12 opted for a simpler version, the “Quick Disabilities of the Arm, Shoulder and Hand” questionnaire (QuickDASH). Uppal et al.10 also reported on pinch and power grip strength, ranges of movement of hand joints, hand span and Kapandji thumb opposition test.
Injection protocolInjection protocols differed between studies, and even within the same study12 (Table 3). From the 7 serotypes of BT existent, 2 were used: type A (BT-A) and type B (BT-B). Motegi et al., 20179 were the only ones that used BT-B (NeuroBloc®). As for the remaining 4 investigation groups, all chose BT-A (BOTOX®); Serri et al.,12 however, used BT-A from 2 different suppliers (BOTOX® and Xéomin®).
Injection protocols performed by each study.
| Study | Type of BT | Injection sites | Units injected |
|---|---|---|---|
| Bello et al., 20178 | A (Botox®) | Dorsal surface in 7 points of hand: 2nd, 3rd, and 4th web spaces, radial side of the index finger base, ulnar side of the small finger base, and each side of the thumb base | 50U/hand |
| Motegi et al., 201611 | A (Botox®) | 2 injections proximally to A1 pulley of the most symptomatic finger of each hand | 20U/finger |
| Motegi et al., 20179 | B (NeuroBloc®) | Palmar surface in 6 points of hand: web spaces, radial side of the thumb base and ulnar side of the small finger base | 3 groups: 250U/hand; 1000U/hand; 2000U/hand |
| Serri et al., 201312 | A (Botox, Xéomin®) | 2 protocols: A with 14 palmar points of hand: 2 at the base of each finger plus 4 in the palmar creases; B with 18 palmar points of hand: 2 at the base of each finger and 2 at the proximal extremity of second phalange of 2nd to 5th finger | 100U/hand |
| Uppal et al., 201410 | A (Botox®) | Palmar surface, at distal palmar crease, in web spaces, around digital neurovascular bundles of all 5 digits of the non-dominant hand (number of points not reported) | 100U/hand |
BT, Botulinum toxin.
Injection sites and units (U) administered were also highly heterogeneous. Bello et al.8 was the only to adopt a dorsal side approach, with the lowest dose of BT-A (50U per hand). Motegi et al., 201611 opted to inject only the most symptomatic finger, through the palmar side, with a total of 20U of BT-A. Remaining authors followed a multiple injection procedure, in the palmar side of hand.9,10,12 Uppal et al. and Serri et al. (in one protocol) also injected the palmar crease besides fingers’ bases.10,12
Follow-upTimings of follow-up were heterogeneous. Considering the potential time of effect of BT, only the two RCT and 1 CS adopted a sufficient time lapse – up to 16 weeks.8,9,11
Studies’ findingsTable 4 resumes the main findings of each report on RP, perfusion, DU, hand function and complications.
Main findings reported by the studies included in the systematic review.
| Study | Raynaud's phenomenon | Digital ulcers | Hand function | Perfusion | Complications |
|---|---|---|---|---|---|
| Bello et al., 20178 | Faster decline of RCS in BT treated arm | No significant results | No significant results | Worse blood flow in BT treated arm at 4 weeks | Transient weakness of intrinsic muscles of hand in 5% of sample (n=2) |
| Motegi et al., 201611 | Improvement in RCS, pain-VAS and skin temperature recovery time | Complete healing of DU | Not evaluated | Not evaluated | Not found |
| Motegi et al., 20179 | RCS:Lower in the 250U-arm vs control at week 4;Lower in the 1000U and 2000U arms vs control group and 250U arm, at weeks 4/8/12/16.Pain VAS:Lower in the 250U-arm vs control at week 8/12;Lower in the 1000U and 2000U arms vs control group, at weeks 4/8/12/16.Skin temperature recovery:Lower in the 2000U-arm vs control and 250U-arm at week 4. | Lower number of DU in the 1000U and 2000U arms vs control group, at week 4/8/12/16 | Not evaluated | Not evaluated | Transient weakness of intrinsic muscles of hand in 2.2% of sample (n=1) |
| Serri et al., 201312 | Improvement in Pain-VAS | Complete healing of all DU, without new ones | Improvement in QuickDASH score | Improvement in oxygen partial pressure | Transient weakness of intrinsic muscles of hand in 16.7% of sample (n=3) |
| Uppal et al., 201410 | No significant results | 75% of patients with DU with complete lesions’ healing | Improvement in pinch grip, power grip, 2nd MCPJ flexion and 3rd MCPJ flexion | Not evaluated | Transient weakness of intrinsic muscles of hand in 10% of sample (n=2) |
BT, Botulinum toxin; DASH, disability of arm, shoulder and hand questionnaire; DU, digital ulcer; MCPJ, Metacarpophalangeal joint; RCS, Raynaud's condition score; VAS, visual analogue scale.
Bello et al. reported a statistically significant faster decline in RCS in the treatment group.8 In Motegi et al., 2016,11 RSC significantly reduced from 4 to 16 weeks after injection. In Motegi et al., 2017,9 RCS in the group treated with 250U was significantly lower than in the control group at week 4; in the groups treated with 1000 and 2000U BT-B, values were significantly lower than those in control group and the group treated with 250U BT-B at every follow-up check-point; groups treated with 1000 and 2000U BTX-B were comparable.
Peripheral perfusionSurprisingly, the RCT with the largest sample8 reported a statistically significant worse blood flow in BT treated arm from baseline to 1 month; however, this result was not verified in the period from baseline to 4 months. No difference was found between groups on Oxygen Saturation.
Distinctly, Serri et al. reported an improvement on Oxygen Partial Pressure (p=0.003) at the end of follow-up.12
Digital ulcersBello et al.8 found no significant differences between treatment and control groups, neither concerning the risk of new ulcers, nor changes over time in number of new ulcers. In Motegi et al., 2016,11 half the sample (n=5) presented DU at the beginning of follow-up; after 16 weeks, every patients’ DUs had healed. In Motegi et al., 2017,9 the numbers of DUs in the groups treated with 1000 and 2000U were significantly lower than those in the control group 4–16 weeks after injection; the same 2 groups had no new DU, in comparison to 2 DU in the group treated with 250U and 7 in the control group. As for Serri et al.,12 10 out of 18 patients had active DU at baseline (median 4 DU per patient); at the end of follow-up, 100% had healed. In Uppal et al.,10 75% of the patients with DU (3 out of 4) had complete lesions’ healing at the end of follow-up.
Hand functionOf the 3 studies that evaluated hand function,8,10,12 2 reported positive results.10,12 Serri et al.12 found a significant improvement in the QuickDASH score. In Uppal et al.,10 objective measures of hand functionality also showed improvement (pinch grip, power grip, 2nd/3rd metacarpophalangeal joint flexion).
ComplicationsBT administration was mostly safe through all studies. The only adverse reaction reported was weakness of hand's intrinsic muscles, with a prevalence ranging between 0 and 16.7% (Table 4). Considering all 5 studies, only 8 out of 133 patients reported this adverse effect (6.0%). Moreover, this was a transitory effect, which reverted completely after the local effect of BT wore of.
DiscussionBT first emerged in the 1970s in the treatment of strabismus, as it prevents muscular contraction by inhibiting the release of acetylcholine in the neuromuscular junctions.13 This mechanism justifies most of the clinical indications of this neurotoxin.14,15 However, evidence suggests the additional role of pain transmission blockage (for example, substance P).16–18 BT also inhibits sympathetic adrenergic vasoconstriction and endothelial exocytosis of endothelin-1,19 through a dose-dependent mechanism, hence justifying the increasing interest in vasospastic disorders.
Our search identified 2 RCT focusing on SSc-related RP/DU. Bello et al.8 presented the most precise study. Unfortunately, it showed the least impressive results and reported a worse blood flow in the treated arm. However, when comparing the units of BT, lower values were administered (versus (10–12)). Considering that the vasodilating effects of BT are shown to be dose-dependent, this might have contributed to the absence of positive results. Despite this, it still reported encouraging findings, with faster decline in RCS in the BT treated arm.
A direct comparison between RCT is unfeasible due to the use of different BT subtypes. Motegi et al., 20179 opted for BT-B instead of BT-A. If one considers an equivalency of 1:40, the highest dose administered of BT-B in Motegi et al., 20179 equalled the 50U of BT-A in Bello et al.8 and still reported significantly better RCS/pain/skin temperature recovery. However, this equivalency ratio is not applicable to the vasodilator effect.13
The only CC included failed to report the selection criteria (Both SSc classification criteria and exclusion criteria).10 It focused mainly on hand function, besides RP and DU, and showed statistically significant improvements in pinch grip, power grip, DASH score and 2nd/3rd metacarpophalangeal joints’ flexion. The 2 CS,11,12 despite the limitations, showed significant improvement of numerous outcomes: RCS,11 pain,11,12 skin temperature recovery time,11 hand function,12 O2 partial pressure12 and DU complete healing.11,12
Concerning DU healing, Motegi et al., 20179 showed statistically significant results, for 1000 and 2000U of BT-B. Bello et al.8 did not support this finding, but all other studies reported positive results, with complete DU healing in 75%10 to 100%11,12 of patients.
Future investigation in this area needs improvement. For example, BT achieves its maximal effect after 2 weeks and then gradually decreases for 2–3 months.13 Thus, follow up time is of crucial importance when reporting results. Only the 2 RCT and 1 CS included a sufficient time-period in their study.8,9,11 This aspect needs to be consistent while evaluating BT in SSc-related peripheral vasculopathy. Environmental conditions must also be reported, specifically daily minimum temperature. Only Bello et al.8 adjusted statistical analysis to this confounding factor. Other studies were performed during the Winter,9,11 when RP/DU are more common and severe.
Another limitation is sample's selection. Some authors did not report the classification criteria applied for patient selection,10,12 and one failed to present the exclusion criteria.10 All of them administered BT as adjuvant measure. This aspect greatly compromises the conclusions drawn, especially because none of the 5 studies included a satisfactory description of baseline vasodilator medications. Moreover, none of the authors performed a description of systemic manifestations with enough accuracy.
The injection protocol was also highly variable. BT-A doses varied between 20U/finger and 100U/hand,8,10–12 and one study opted for BT-B.9 Injection sites differed markedly, with 1 group using a dorsal approach8 and 2 injecting the palmar creases.10,12 In the future, researchers should focus mainly on only one BT type, and perform similar injection procedures (for example, using the palmar approach, but avoiding injecting the palmar crease, because of a possible higher risk of muscle weakness12).
Outcome measures must also be uniformized. RCS is the only tool validated for RP assessment.20 Other possibilities to include are frequency/duration of RF attacks and VAS of patient's and physician's assessment of RP activity. DU, namely the number of ulcers and development of new ulcers, should be systematically reported. Hand function assessment is a secondary outcome that can be included – DASH or QuickDASH questionnaires showed validity in SSc21 but other tools are available.22
Previous reviews focusing on this subject have been performed, with promising findings.7 However, the studies included primary and secondary FP of different aetiologies, thus invaliding conclusions concerning only SSc. This has impact in daily clinical practice, because the most severe cases of peripheral vasculopathy are typically associated to SSc. In light to this scenario, this review clarifies that BT can also claim a role in this population.
ConclusionDespite not conclusive, evidence suggests that BT has a position to claim in the treatment of SSc-related vasculopathy – it may not be necessarily an anchor therapy, but an effective and safe adjuvant to the vasodilating drugs presently recommended in the treatment of RP/DU. However, in face of conflicting results of one RCT, more robust studies are needed to clarify its true efficacy, as well as the optimal dose and injection protocol.
Key points- •
Treatment of peripheral vasculopathy (RP/DU) in SSc can pose a difficult challenge in clinical practice.
- •
BT, through inhibition of sympathetic adrenergic vasoconstriction and endothelin-1, has emerged as an alternative treatment in this scenario.
- •
Current evidence supports a positive effect of BT on RP severity and DU healing, but is held back by several methodological limitations of the studies performed to date.
- •
Future investigation is required to further clarify the findings of this review, especially the conflicting results of 1 RCT.
- •
For the moment, BT can be considered an adjunctive in the treatment of refractory RP/DU, but not an anchor therapy.
MG – Ideation of the study, substantial contributions to the design of the study, acquisition of data, analysis and interpretation of data, drafting of the article, critical revision of the intellectual content, final approval of the version to be published.
DF – Acquisition of data, substantial contributions to the design of the study, acquisition of data, analysis and interpretation of data, critical revision of the intellectual content, final approval of the version to be published.
BS – Substantial contributions to the design of the study, analysis and interpretation of data, critical revision of the intellectual content, final approval of the version to be published.
TV – Ideation of the study, substantial contributions to the design of the study, critical revision of the intellectual content, final approval of the version to be published.
PP – Ideation of the study, substantial contributions to the design of the study, critical revision of the intellectual content, final approval of the version to be published.
FundingNo funding received.
Conflict of interestNone to declare.