To evaluate the usefulness of serum concentrations (Sc) of adalimumab (ADA) as a predictor of medication adherence using the medication possession ratio (MPR) and Morisky Green test (MGT) in patients with chronic inflammatory diseases.
Material and methodDesign a prospective descriptive cohort study. Inclusion criteria: adult patients diagnosed with inflammatory arthropathy (IA) or inflammatory bowel disease (IBD) treated with ADA. Exclusion criteria: positive anti-adalimumab antibody. Variables: sex, age, diagnosis, dosage regimen, Sc (mg/ml), MPR (MPR ≥80% adherent) and MGT (non-adherent or adherent). Statistical analysis was performed using STATA v13.0.
ResultsForty-five patients (23 women) with an age of 52.22 (14.39) years, 17 IBD (37.78%), 26 IA (57.78%) and 2 with both conditions (4.44%) treated with 40mg ADA every 14 days (42/45; 93.33%) or every 7 days (3/45; 6.67%).
We detected subtherapeutic Sc in 22.22% of patients (10/45); 10% (1/10) were classified as non-adherent and 90% (9/10) as adherent according to MGT and MPR.
The quantification of Sc shows weak agreement with MPR, as was the case with the indirect methods of each (MPR and MGT). The association was slightly greater when the indirect methods were compared to each other (0.244 vs 0.378).
ConclusionThe determination of Sc of ADA alone has limited utility in the detection of non-adherent patients.
Evaluar la utilidad de la determinación de concentraciones séricas (Cs) de adalimumab (ADA) como factor predictor de la adherencia al fármaco medida a través de la tasa de posesión de medicación (TPM) y del test de Morisky Green (MG) en pacientes con enfermedades crónicas inflamatorias.
Material y métodoDiseño prospectivo, descriptivo de cohortes. Criterios de inclusión: pacientes adultos con artropatías inflamatorias (AI) o enfermedad inflamatoria intestinal (EII) en tratamiento con ADA. Criterios de exclusión: pacientes con anticuerpos anti-ADA. Variables: sexo, edad, diagnóstico, pauta posológica, Cs (μg/ml), TPM (TPM ≥80% adherentes), y resultado del test de MG (no adherente o adherente). El análisis estadístico se realizó mediante STATA v13.0.
ResultadosCuarenta y cinco pacientes (23 mujeres) con edad de 52,22 (14,39) años, 17 EII (37,78%), 26 AI (57,78%) y 2 con ambas enfermedades (4,44%) tratados con ADA cada 14 días (42/45,93,33%) o cada 7 días (3/45;6,67%).
Se detectaron Cs infraterapéuticas en el 22,22% pacientes (10/45): el 10% (1/10) se clasifican como no adherentes y el 90% (9/10) como adherentes según MG y TPM.
La Cs con la TPM, así como los métodos indirectos entre sí (TPM y MG) presentaron un índice de acuerdo débil, siendo la asociación ligeramente superior al relacionar los métodos indirectos entre sí (0,244 vs 0,378).
ConclusiónLa determinación de Cs de ADA presenta, por sí sola, una utilidad limitada en la detección de pacientes no adherentes.
Inflammatory arthropathies (IA) and inflammatory bowel disease (IBD) are relatively highly prevalent in the population. They are chronic and debilitating, and greatly impact the quality of life and functionality of the people who suffer them.1 There has been a revolution in the management of these diseases with the introduction of biological therapies, particularly the tumour necrosis factor (TNF) alpha blocking drugs (anti-TNF-α), that are frequently self-administered subcutaneously by patients, in weekly, fortnightly, etc. dosage regimens.
According to various authors, adherence is considered crucial in treatment response, associated with high rates of effectiveness2,3 and, therefore, has a direct impact on the control of these chronic diseases. Failure to adhere to treatment is a priority issue for the World Health Organisation (WHO), because it is so highly prevalent and its association with poorer control of the disease, increased morbidity and mortality,4 reduced quality of life and increased healthcare costs.5 Adherence rates vary considerably in the different studies,6 between 70% of patients with rheumatoid arthritis (RA)7 and 85% with Crohn's disease (CD).8 Likewise, maximum adherence is observed when chronic treatments are started, but this tails off the longer the treatment continues.9 A review undertaken by the Cochrane10 collaboration concluded that improved adherence to treatment could even have a greater impact on clinical outcomes than treatment with a new innovative drug. Aware of the relevance of this key factor, interdisciplinary teams that include hospital pharmacists, are undertaking campaigns to promote patient co-responsibility for their health care (self-care, adherence, etc.), in line with the conclusions of the MAPEX project.11
There is currently no single method considered the benchmark in measuring adherence, therefore several should be used to mitigate the limitations that they all display.12 The available methods are as follows: (1) direct objective methods, based on the quantification of a drug as a biological fluid by testing Sc of ADA; (2) indirect object methods, such as the medication possession ratio (MPR), and (3) indirect subjective methods, based on clinical interview techniques using questionnaires such as the Morisky Green (MG) test.13
To date there has been little evidence for the relationship between the different methods available for measuring adherence. Therefore this study aims to assess the clinical utility of testing serum concentrations (Sc) of adalimumab (ADA) as a predictor of adherence to medication using the MPR and the MGT, and the concordance between both indirect methods in patients with chronic inflammatory diseases.
Material and methodsA prospective descriptive cohort study performed in a public, general university hospital belonging to a health department covering 270,000 inhabitants, with 129 patients treated with biological therapies prescribed for IBD and IA in 2015. The study duration was 16 months: from November 2014, when the Sc testing of biological drugs was implemented, until February 2016.
Adult patients diagnosed with IA (RA, psoriatic arthritis [PsA] or ankylosing spondylitis [AS]) or IBD (Crohn's disease [CD] or ulcerative colitis [UC]) under treatment with ADA for a minimum of 6 months, and candidates for Sc testing based on criteria of effectiveness, safety, efficacy or suspected lack of adherence to treatment according to the judgement of the prescribing physician over the study period.
Patients whose adherence had not been validated using the MG test, because it was not available at the time the sample was extracted, and those with positive anti-ADA antibodies (>10ng/ml) were excluded from the study. For patients who had undergone 2 Sc tests over the study period, only the result of the variables studied in the first test was included.
The demographic variables collected were sex and age, and diagnosis and variables relating to treatment: line of biological treatment, time treated with ADA, dosage regimen and concomitant immunomodulator (IMM) treatment, 5-aminosalicylic acid derivatives (5-ASA) or glucocorticoids.
Three methods were used to assess adherence:
- 1)
Sc testing (direct objective): obtaining Sc of ADA (μg/ml) using the ELISA test (Promonitor®, Progenika Biopharma Grifols S.A., Spain). The therapeutic interval (TI) was established as: 5–8μg/ml14 for IA and 6–8μg/ml15 for IBD. Levels below 5μg/ml for patients with IA, and below 6μg/ml for patients with IBD were considered subtherapeutic.
- 2)
MPR (indirect objective method) in the 6 months prior to Sc testing, calculated as: number of units dispensed×dosing interval (days)/days between dispensing over a period of approximately 6 months prior to the time of monitoring×100 (%). Patients considered adherent to treatment were those with an MPR ≥80%, and those considered non-adherent those with an MPR <80%. The clinical histories of the latter were checked for any reasons that would have impeded administration of the treatment in the 6 months prior to monitoring (e.g. contraindication due to a serious infection).
- 3)
MG test (indirect subjective method) at the time of monitoring. Patients considered non-adherent to treatment were those who answered “yes” to at least one of the following questions: (a) “Have you ever forgotten to take the medication to treat your disease?”; (b) “Do you forget to take the medication at the prescribed times?”; (c) “When you feel well, do you stop taking your medication?”, and (d) “If you ever feel unwell, do you stop taking your medication?”.
The sources of information were: the Sc test results recorded in the i-GestLab® (Cointec Ingenieros y Consultores, S.L.), the record of treatment dispensations collected from the pharmacotherapeutic history in the Farmasyst® application, the data collection logbook with the MG test, completed by the prescribing physician and nursing staff, and the electronic clinical history of the Orion Clinic® application (Everis Spain S.L.U.).
The categorical variables were described in frequencies (%), and the quantitative variables by mean and standard deviation (SD). The Sc test results were studied as a dichotomous variable: subtherapeutic or within the TI/supratherapeutic, and compared with the MG (%) and the MPR (%) result, also as dichotomous variables, using Pearson's χ2 test. We then compared the mean Sc for each of the groups according to the MG test (%) and the MPR (%) result, using the Student's t-test.
The percentages of patients with subtherapeutic Sc and non-adherent were determined, according to sex (compared using Person's χ2 test), age and time under treatment (using the Student's t-test).
A level of significance of at least .05 was considered for all the statistical tests. STATA v13.0 software was used for the statistical analysis.
Overall, positive and negative specific agreement indices were used to study the agreement rates between the different measurement methods, and the PABAK index (prevalence-adjusted and bias-adjusted kappa) that considers: <.10 no agreement, .11–.40 weak, .41–.60 slight, .61–.80 moderate, and .81–1 perfect agreement.16,17
This study complied with the Declaration of Helsinki and its later revisions. All the participating patients were duly informed, and completed and signed their informed consent forms in duplicate. The investigators allocated each of their patients an identification code to maintain data confidentiality in compliance with current legislation (RD 1720/2007, enacting Organic Law 15/1999, of 13 December, on the Protection of Personal Data). The project was approved by the hospital's Clinical Research Ethics Committee.
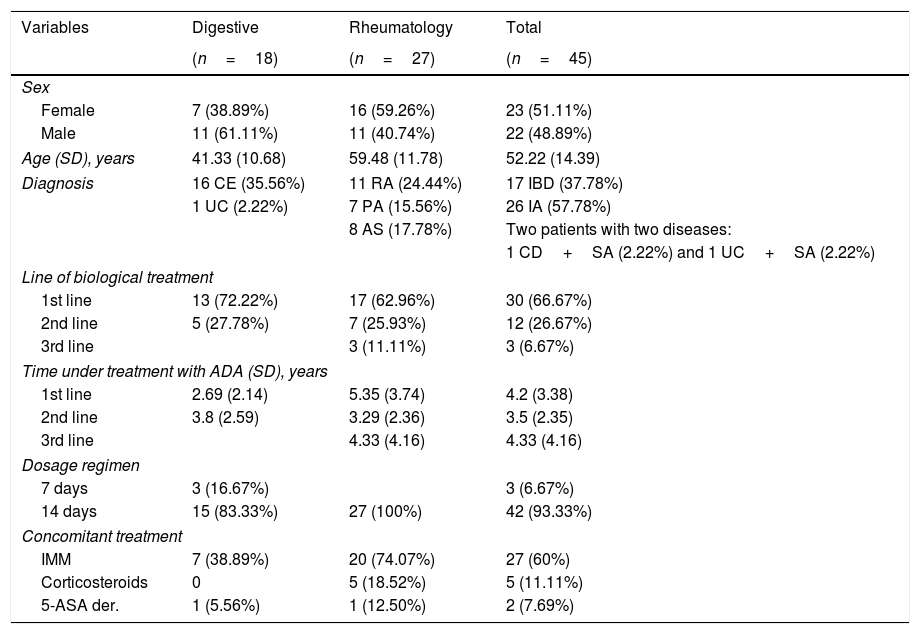
ResultsFifty patients were recruited through the 16 months of the study; 5 patients had anti-ADA antibodies (>10ng/ml), and were therefore excluded from the study. The demographic, diagnostic, and treatment-related characteristics of the 45 patients included are summarised in Table 1.
Demographic, diagnostic and treatment-related variables.
| Variables | Digestive | Rheumatology | Total |
|---|---|---|---|
| (n=18) | (n=27) | (n=45) | |
| Sex | |||
| Female | 7 (38.89%) | 16 (59.26%) | 23 (51.11%) |
| Male | 11 (61.11%) | 11 (40.74%) | 22 (48.89%) |
| Age (SD), years | 41.33 (10.68) | 59.48 (11.78) | 52.22 (14.39) |
| Diagnosis | 16 CE (35.56%) | 11 RA (24.44%) | 17 IBD (37.78%) |
| 1 UC (2.22%) | 7 PA (15.56%) | 26 IA (57.78%) | |
| 8 AS (17.78%) | Two patients with two diseases: | ||
| 1 CD+SA (2.22%) and 1 UC+SA (2.22%) | |||
| Line of biological treatment | |||
| 1st line | 13 (72.22%) | 17 (62.96%) | 30 (66.67%) |
| 2nd line | 5 (27.78%) | 7 (25.93%) | 12 (26.67%) |
| 3rd line | 3 (11.11%) | 3 (6.67%) | |
| Time under treatment with ADA (SD), years | |||
| 1st line | 2.69 (2.14) | 5.35 (3.74) | 4.2 (3.38) |
| 2nd line | 3.8 (2.59) | 3.29 (2.36) | 3.5 (2.35) |
| 3rd line | 4.33 (4.16) | 4.33 (4.16) | |
| Dosage regimen | |||
| 7 days | 3 (16.67%) | 3 (6.67%) | |
| 14 days | 15 (83.33%) | 27 (100%) | 42 (93.33%) |
| Concomitant treatment | |||
| IMM | 7 (38.89%) | 20 (74.07%) | 27 (60%) |
| Corticosteroids | 0 | 5 (18.52%) | 5 (11.11%) |
| 5-ASA der. | 1 (5.56%) | 1 (12.50%) | 2 (7.69%) |
ADA: adalimumab; IA: inflammatory arthropathies; PsA: psoriatic arthritis; RA: rheumatoid arthritis; UC: ulcerative colitis; SD: standard deviation; 5-ASA der.: 5-aminosalicylic acid derivatives; SA: ankylosing spondylitis; CD: Crohn's disease; IBD: inflammatory bowel disease; IMM: immunomodulators.
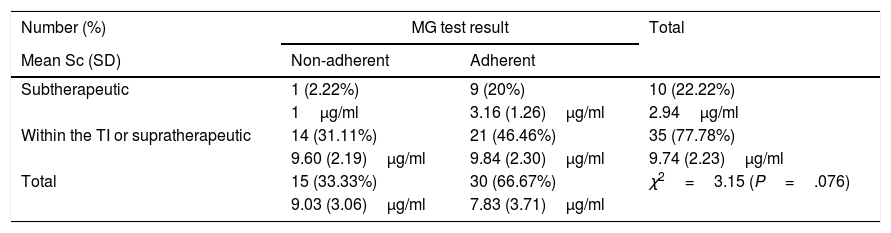
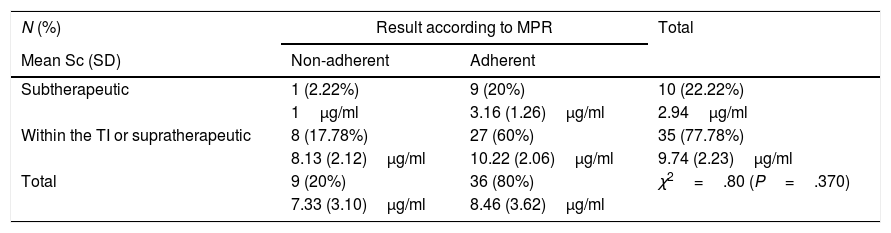
Subtherapeutic levels of ADA were detected in 22.22% (10/45) of the patients, of whom only one (1/10; 10%) was considered non-adherent according to the MG test (χ2=3.15; P=.076), and the MPR (χ2=.80; P=.370), as described in Tables 2 and 3. Within this subgroup, Sc were lower in the patient classed as non-adherent (Sc 1μg/ml) compared to those classed as adherent (3.16 [1.26]μg/ml) using either of the 2 indirect methods.
Results of Sc testing (percentage of patients and plasma levels measured with standard deviation) and of the MG test.
| Number (%) | MG test result | Total | |
|---|---|---|---|
| Mean Sc (SD) | Non-adherent | Adherent | |
| Subtherapeutic | 1 (2.22%) | 9 (20%) | 10 (22.22%) |
| 1μg/ml | 3.16 (1.26)μg/ml | 2.94μg/ml | |
| Within the TI or supratherapeutic | 14 (31.11%) | 21 (46.46%) | 35 (77.78%) |
| 9.60 (2.19)μg/ml | 9.84 (2.30)μg/ml | 9.74 (2.23)μg/ml | |
| Total | 15 (33.33%) | 30 (66.67%) | χ2=3.15 (P=.076) |
| 9.03 (3.06)μg/ml | 7.83 (3.71)μg/ml | ||
Sc: serum concentration; SD: standard deviation; TI: therapeutic interval; MG: Morisky Green test.
Results of Sc testing (mean percentage of patients and plasma levels with standard deviation) and MPR.
| N (%) | Result according to MPR | Total | |
|---|---|---|---|
| Mean Sc (SD) | Non-adherent | Adherent | |
| Subtherapeutic | 1 (2.22%) | 9 (20%) | 10 (22.22%) |
| 1μg/ml | 3.16 (1.26)μg/ml | 2.94μg/ml | |
| Within the TI or supratherapeutic | 8 (17.78%) | 27 (60%) | 35 (77.78%) |
| 8.13 (2.12)μg/ml | 10.22 (2.06)μg/ml | 9.74 (2.23)μg/ml | |
| Total | 9 (20%) | 36 (80%) | χ2=.80 (P=.370) |
| 7.33 (3.10)μg/ml | 8.46 (3.62)μg/ml | ||
Sc: serum concentration; SD: standard deviation; TI: therapeutic interval; MPR: medication possession rate.
When stratifying according to the number of responses that defined patients as non-adherent or adherent as per the MG test (15/45; 33.33%), 86.67% (13/15) were classed as non-adherent having answered “yes” to one of the questions, while the remaining 13.33% (2/15) replied in the affirmative to 2 or 3 questions. The distribution of each of these 18 affirmative answers was: question b 61.11% (11/18), question a 27.78% (5/18), question c 5.56% (1/18), and question d 5.56% (1/18). The only patient who gave 3 affirmative answers in the MG test had subtherapeutic levels of ADA.
After stratifying by sex, 5 women (21.74%) and 5 men (22.73%) showed subtherapeutic Sc; similar results to those provided by the MPR; 4 women (17.39%) and 5 men (22.73%) were considered non-adherent using this method. According to the MG test, 26.67% (4/23) of the women and 50% (11/22) of the men were considered non-adherent (χ2=5.380; P=.020). Neither the age of the patient nor the time of treatment with the drug were related to the different adherence measurement results, except the relationship between treatment time with ADA and the MPR, which was 7.11 (3.14) years of treatment with ADA in the non-adherent group vs. 3.25 (2.64) years in the adherent group (t=3.776; P=.001).
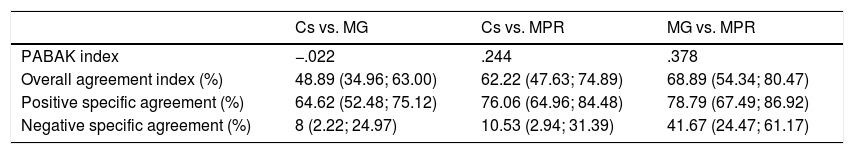
The agreement rates between the different adherence measurement methods are detailed in Table 4. Sc with the MPR, and the indirect methods between each other (MPR and MG) showed a weak agreement rate; the association was slightly higher when comparing the indirect methods with each other (.244 vs. .378), which coincides with higher overall and specific agreement rates.
Results of the indices according to the different adherence measurement methods.
| Cs vs. MG | Cs vs. MPR | MG vs. MPR | |
|---|---|---|---|
| PABAK index | −.022 | .244 | .378 |
| Overall agreement index (%) | 48.89 (34.96; 63.00) | 62.22 (47.63; 74.89) | 68.89 (54.34; 80.47) |
| Positive specific agreement (%) | 64.62 (52.48; 75.12) | 76.06 (64.96; 84.48) | 78.79 (67.49; 86.92) |
| Negative specific agreement (%) | 8 (2.22; 24.97) | 10.53 (2.94; 31.39) | 41.67 (24.47; 61.17) |
Sc: serum concentration; MG: Morisky Green test; MPR: medication possession rate.
It is evident that adherence affects the effectiveness of drugs. However, it is difficult and controversial to measure, since there is no ideal assessment method; therefore, establishing the best adherence measurement method for chronic patients is a fundamental goal for health professionals. In a systematic review performed in 2011 on patients with RA, according to the authors, the adherence measurement methods were unclear and varied greatly among the different studies included in the review,18 as a result of the different concepts of adherence that each evaluated.
It has been suggested that the best approach is to combine direct and indirect measurement methods, which, when used simultaneously minimises limitations and increases accuracy.19 In this study we assessed the relationship between adherence to the drug measured using a direct method (Sc), and 2 indirect methods (MG test and MPR), in order to determine the clinical utility of ADA Sc testing as a predictor of adherence to treatment.
Adherence measurement, ideally assessed using a combination of direct and indirect methods, should form part of the pharmacotherapeutic plan for patients under treatment with biological drugs due to the chronic nature of the disease, the risk of developing antibodies, and the high cost of these types of therapies. Using these methods to evaluate adherence, and assessing clinical response, will enable the identification of non-adherent patients and multi-disciplinary intervention to avoid compromising the effectiveness, safety and efficiency of the treatment. As other authors have already highlighted, a broader validation of the different measurement methods is highly desirable, with a view to defining standards to facilitate adherence monitoring and optimise interventions in clinical practice.18
Sc testing of biological therapies has recently been included in clinical practice in order to tailor dosage regimes according to each individual patient's characteristics, with a positive impact on the effectiveness, safety14,20 and efficacy of treatment.21 It is likely that a direct adherence measurement method would be more objective and truthful than the indirect methods, based on patient statements; however, no study has suggested analysing the clinical utility of Sc testing of biological therapies as a direct method, complementing other indirect adherence measurement methods. However, the relationships have been studied between direct and indirect adherence measurement methods (dispensation records) for other drugs, such as phenytoin and digoxin. Significant results were obtained comparing the Sc of both drugs with continuous adherence measurements, with correlation coefficients between .2 and .4,22,23 similar results to those obtained in our hospital (.244) despite the categorisation of the variables used.
In our study, the MG test and the MPR showed a weak agreement rate (.378) but better than that obtained when Sc were compared to the indirect methods. Of these, the association between Sc and MPR was higher (.244), which indicates to us that MPR is more reliable in identifying non-adherent patients.
Among the limitations of the direct adherence measurement methods, we must bear in mind that Sc can be obtained within the TI for non-adherent patients, because Sc testing of a single sample, as in our study, provides information on recent adherence to treatment and not on the entire therapeutic regimen. Similarly, subtherapeutic Sc can be obtained in adherent patients due to: pharmacokinetic and pharmacodynamic processes, the technique for detecting the drug, benchmark TI, etc., which are also important sources of variability, which affect the results of monitoring.24
According to some authors, the rate of patients with subtherapeutic Sc of ADA in Rheumatology is 35.1% (excluding the percentage of patients with positive anti-ADA antibodies).25 These results are even higher than those obtained in our centre (22% of patients with subtherapeutic Sc). We must also take into account that ADA has high interindividual variability26 which, according to the summary of product characteristics,27 could require dose optimisation or intensification to achieve maximum effectiveness of the treatment.
With regard to the use of indirect adherence measurement methods, and in relation to the use of the MPR, we must consider that dispensing the medication from the hospital pharmacy does not mean correct compliance with the therapeutic regimen, therefore this method tends to overestimate adherence. In a study performed on patients with RA treated with ADA, an MPR ≥80% was only achieved in 67% of the population,28 while adherence defined as “neither delaying nor missing a dose in the last 3 months” was 55% in a study on patients with CD.29 Dispensing frequencies might be one of the reasons for these differences, if we consider that a greater number of dispensations could improve physician–patient contact, and therefore adherence to medication.30
Adherence measurement quantified by the dispensation registry indicated that 90% of patients with subtherapeutic Sc of ADA were adherent, with no important differences in terms of sex, age and treatment time, and the same result was obtained using the MG test. In studies comparing different indirect adherence measurement methods, such as the SMAQ and the MPR (considering people with an MPR ≥90% adherent), the results obtained showed up to 20% discordance between the two measures.31 If we compare the adherence results of the MG test with an MPR ≥95%, the values are similar in the total number of patients included (66.67% vs. 51.11%). This similarity is reasonable, bearing in mind that the MG was validated in Spanish for hypertensive patients who were required to have an MPR of between 80% and 100%, with a sensitivity of 52%, and specificity of 44.4%.13
Neither do we know how truthfully each patient answers adherence assessment questionnaires, generally administered by health staff, and always processed with through recall and response biases. In a prospective study on RA patients who underwent adherence assessment using the Compliance Questionnaire for Rheumatology, adherence measured at 6 months was 74.99% (10.40%).32 Other authors obtained 68.7% adherence using this same test on patients with osteoporosis attended in primary care,33 similar results to those of our study (66.67%). The results of this questionnaire have also recently been correlated in our country with those of the MG test, obtaining weak agreement rates between the two indirect methods (.186),34 lower than those obtained in our hospital when we correlated them with the MPR (.378).
It is likely that the factors that bias the diverse adherence measurement methods, which include the evaluation of a single Sc, and the small sample size, determined the weak agreement rates obtained. It would also have been desirable to study adherence for each of the diseases individually, and of patients with a similar clinical situation, since the MG test assesses whether patients have an appropriate attitude to the treatment,35 and this can be determined by their underlying disease and their condition at the time of performing the test.
It is noteworthy that, for reasons other than the presence of antibodies, subtherapeutic levels were explained by a lack of adherence to treatment in only 10% of the patients, whereas in the remaining 90% these Sc were determined by the drug's high interindividual variability. This might result in less likelihood of achieving clinical remission or minimal disease activity in clinical practice,36 leading to individualised modifications of dosage regimens or changes of biological drug. In this context, testing Sc of ADA is a very useful tool for decision-making, due to the strong relationship between levels of the drug and antibodies and clinical response.37 As long as there are pharmacokinetic-pharmacodynamic population models to manage the interindividual variability of the biological drugs, including their molecular targets, such as TNF-α, we can distinguish whether a patient who shows subtherapeutic levels is due to populational variability or other factors such as adherence, which is the purpose of this study.
To conclude, in our study testing Sc of ADA with indirect adherence measurement methods showed a weak agreement rate, the association was slightly higher when the indirect methods were compared to each other. Therefore testing Sc of ADA on its own is of limited clinical utility in detecting patients with IA and IBD who are not adhering to treatment. Therefore, only when there is a prior suspicion of non-adherence might Sc testing be a useful tool to confirm or rule out this possibility, and then use educational and behavioural measures to improve treatment adherence.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sáez Belló M, Llopis Salvia P, Alegre Sancho JJ, Paredes Arquiola JM, Asencio Muñoz MC, Climente Martí M. Utilidad de las concentraciones séricas de adalimumab como predictores de adherencia al tratamiento. Reumatol Clin. 2020;16:32–37.