The relevance of polyautoimmunity, defined as the presence of two or more autoimmune diseases in the same individual, is one of the issues not yet elucidated in medical practice. The coexistence of myasthenia gravis (MG) and systemic lupus erythematosus (SLE) is a clinical challenge due to the possible differential diagnoses of muscle involvement in patients with SLE. We present the case of a patient who came to the emergency room of Hospital Universitario San Ignacio in Bogotá, Colombia, with a previous diagnosis of SLE, who developed acute weakness in the context of a systemic infection, with a clinical and electrophysiological diagnosis of MG.
La relevancia clínica de la poliautoinmunidad, definida como la presencia de dos o más enfermedades autoinmunes en un mismo individuo, es uno de los temas aun sin dilucidar en la práctica médica. La coexistencia entre miastenia gravis (MG) y lupus eritematoso sistémico(LES) supone un reto clínico por los posibles diagnósticos diferenciales dados al momento de abordar el compromiso muscular en pacientes con LES. Presentamos el caso de una paciente que consultó a urgencias del Hospital Universitario San Ignacio de Bogotá, Colombia, con diagnóstico previo de LES, que desarrolla un síndrome de debilidad aguda en el contexto de una infección sistémica, haciendo diagnóstico clínico y electrofisiológico de MG.
The autoimmune diseases (AD) correspond to a heterogeneous group of entities in which a loss of immunological self-tolerance occurs, manifesting clinically as organ-specific or systemic compromise.1 The presence of similar physiopathological mechanisms associated with genetic factors explains the theory of polyimmunity, defined as the presence of 2 or more AD in a single individual. The work by Anaya et al. concludes that there are autoimmunity “chaperones”, and that a history of familial autoimmunity supports the hypothesis that it is not fortuitous.1,2
Myasthenia gravis (MG) is an organ-specific AD that is characterised by dysfunction of the neuromuscular link secondary to the presence of antibodies, with clinical manifestations such as a tendency to fatigue and fluctuating muscle weakness.3 The thymus plays a central physiopathological role in this entity due to the presence of antigens and the activation of T and B cells that correlate with the production of acetylcholine receptor antibodies. It has two forms of presentation: the first peak occurs at from 20 to 30 years old and predominates in women, while the second peak occurs after the age of 60 years old and predominates in men.4
On the other hand, systemic lupus erythematosus (SLE) is one of the AD per excellence, and it is more prevalent in young women. It leads to systemic compromise due to the production of antibodies, the deposit of immune complexes and the activation of the complement cascade, leading to multiple organ damage.5
The frequency with which MG and SLE coexist is variable, and reported rates of incidence run from the 3.78% of patients with SLE reported by Bekircan-Kurt et al., in a cohort with MG, up to 7.7%, with a higher prevalence among women.6 A prevalence of polyautoimmunity is reported of up to 15% in cohorts of patients with MG.7,8
Taking the above considerations into account, we present the case of a patient in whom these entities were confirmed to coexist.
Clinical caseA previously healthy mixed race female patient aged 24 years, from Ibagué, Colombia, who in August 2016 was diagnosed extra-institutionally with SLE by 1:160 ANA indirect immunofluorescence, debuting with the following clinical manifestation: serous involvement, arthritis, anaemia, alopecia, convulsive crises and swiftly progressing glomerulonephritis, requiring support with renal replacement haemodialysis one month after diagnosis. She received outpatient treatment with prednisolone 30mg/day, without taking a renal biopsy, and the rest of her autoimmunity profile was unknown (ENA, anticardiolipins, lupus anticoagulant). After 3 months she required hospitalisation once again in another institution in the intensive care unit (ICU) due to respiratory failure secondary to pneumonia and an episode of acute weakness that was gradually resolved with systemic steroid to treat the SLE, without performing additional studies.
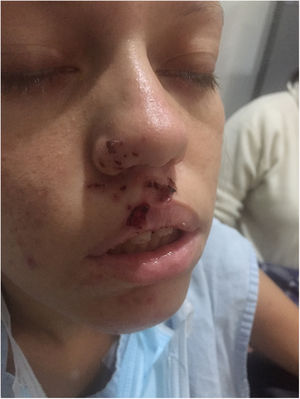
She was referred to the Emergency department of our institution due to symptoms that had evolved over 10 days, with a cough and purulent expectoration associated with fever that was not quantified, shivering, tachycardia and the appearance of vesicular-type lesions on the upper lip and right nasal ala. The day she was admitted she had a focal crisis with alteration in attention and self-limiting bilateral spreading rigidity, so that treatment with lacosamide commenced.
At admission she had raised arterial pressure (204/115mmHg), tachycardia (115 lpm), evidence of ulcerated vesicular lesions on the upper lip and right nasal ala and slight rale in the base of the right lung without signs of respiratory difficulty. Neurological examination showed reduction in the visual acuity of the left eye, explained by serous central choroidopathy, without other findings.
Paraclinical tests at admission found anaemia with haemoglobin at 9.6mg/dl, platelets at 196,500×106, urine analysis with no active sediment, renal ultrasound scan with cortical atrophy, so that it was not possible to characterise the type of lupus nephritis, complement and anti-DNA within normal limits, ruling out lupus activity, with normal thyroid function. Consolidation of the middle lobe was confirmed, ruling out additional complications or other abnormal findings by means of high resolution thoracic tomography. Antibiotic treatment was commenced with clarithromycin and piperacillin tazobactam for pneumonia with risk factors for resistant germs, due to a recommendation by Infectiology, as well as acyclovir for the presence of skin lesions compatible with herpes zoster infection.
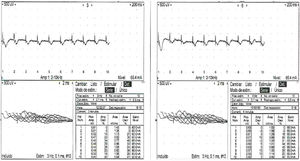
Three days after admission fluctuating episodes of predominantly left side bilateral ptosis were detected (Fig. 1), positive ice pack test, positive fatigability test, eye movements with left supraduction deficit, vertical binocular diplopia, moderate facial diparesia, hypophonia, neck flexoextensor weakness in 2/5 and counted up to 4, without apendicular muscle involvement or other findings in the neurological examination. Due to the presence of these manifestations involvement of the neuromuscular junction was suspected, and the repetitive stimulation test was performed (Fig. 2), showing a post-synaptic disorder in the neuromuscular junction.
The course of a myasthemic crisis was then considered, triggered by the community-acquired pneumonia, with the antecedent of SLE, thereby forming a polyautoimmunity syndrome in which the lack of SLE activity was striking. She was transferred to the ICU due to the risk of respiratory failure and stayed there for 8 days, completing 5 plasma replacement therapy sessions with no complications and resolution of the myasthenic crisis 15 days after the start of symptoms and treatment. She was discharged with immunomodulator treatment with a corticoid, azathioprine and an antimalarial drug, together with symptomatic treatment with pyridostigmine, with the indication to test for anti-acetylcholine receptor antibodies; however, the patient did not attend the following check-ups.
DiscussionThe coexistence of MG and SLE represents a clinical challenge due to the possible differential diagnoses that may be involved when confronted by muscle involvement in patients with SLE. Myopathies in patients with SLE may be manifestations of this disease, or they may be associated with other autoimmune diseases, especially polymyositis, dermatomyositis, thyroid diseases, myotoxicity due to medication and, less frequently, they may be associated with MG as an expression of polyautoimmunity.9
A large proportion of the studies that report on the association between SLE and MG cover cohorts of patients with MG, evaluating polyautoimmunity with other AD.1,6,7 Works such as the one by Rojas-Villarraga et al., which seeks to evaluate factors associated with polyautoimmunity in SLE, do not identify any clear association with MG10; nevertheless, a study performed in Norway in 1984 found that of 48 patients diagnosed with MG, 11 patients had another AD, of whom 4 patients (8.3%) were diagnosed SLE.11 The description by Tanovska et al. shows that 15% of the patients diagnosed MG had another AD, the most frequent of which was autoimmune thyroid disease, this being one of the “chaperone diseases” described by Anaya et al. Bekircan-Kurt et al. reported that 73.3% of the patients with another AD had been diagnosed before MG itself, and in our report the diagnosis of SLE preceded that of MG.1,6,8
The data vary respecting the primary disease, with a prevalence of up to 1.3% of MG in patients with SLE, and up to 8% of SLE in patients with MG; nevertheless, the high prevalence in women was the common factor. The group of Jallouli et al. showed that the clinical manifestation of SLE was less severe in those who were also diagnosed MG.12
Patients who are treated by thymectomy for MG have widely been described to possibly have a predisposition for subsequently developing SLE; this may be due to a loss of central tolerance and excess production of antibodies; however, to date there has been no research to study this relationship in greater depth.4
Another important and interesting phenomenon in autoimmunity is familial aggregation. This has been described in all of the AD, and MG is not an exception. In GLADEL cohort patients as well as in patients with MG, familial autoimmunity has been found to be a risk factor for developing the same AD as well as for other types of AD.1,10,13,14 The work by Liu et al. in Taiwan, in an individual the RR with a first degree family member affected by MG was 2.18 (1.53–3.12) for SLE.14
Although it is not common for these disorders to present together, in different cases it has been possible to see an association between SLE and MG, so that it is important to think of MG as a differential diagnosis when an individual with SLE has symptoms of fluctuating muscle weakness and fatigability.11 Confirming polyimmunity and familial aggregation is therefore clinically relevant for differential diagnosis and proper management of its manifestations and complications.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the members of the departments of Internal Medicine and Neurology for their remarks, which enriched this work.
Please cite this article as: García-Alfonso C, Bernal-Macías S, García-Pardo Y, Patricia Millán S, Díaz MC. Coexistencia de lupus eritematoso sistémico y miastenia gravis. Una expresión infrecuente de poliautoinmunidad. Reumatol Clin. 2020;16:502–505.