COVID-19 is a newly emerged disease that has become a global public health challenge. Due to a lack of knowledge about the virus, a significant number of potential targets for using a particular drug have been proposed. Five cases with a clinical history of biopolymers in the gluteal region that developed iatrogenic allogenosis (IA) are presented here. The 5 cases were put under colchicine treatment for IA crisis and had non-specific symptoms (headache, cough without dyspnea, and arthralgias) with a positive SARS-CoV-2 test. Their close contacts had mild to severe symptoms and three of them died. In the SARS-CoV-2 infection different inflammatory pathways are altered where colchicine reduces cytokine levels as well as the activation of macrophages, neutrophils, and the inflammasome. The possible mechanisms that colchicine may use to prevent acute respiratory distress syndrome (ARDS) in patients with COVID-19 infection are also reviewed in this article.
COVID-19 es una enfermedad de aparición reciente, que se ha convertido en un reto global de salud pública. Debido a la falta de conocimiento acerca del virus, se ha propuesto un número significativo de objetivos potenciales para utilizar un fármaco en particular. Presentamos 5 casos con historia clínica de biopolímeros en la región glútea, que desarrollaron alogenosis iatrogénica (AI). A los 5 casos se les administró tratamiento de colchicina debido a la crisis de AI, no teniendo síntomas específicos (cefalea, tos sin disnea y artralgias), con resultado positivo en el test de SARS-CoV-2. Sus contactos cercanos tenían síntomas de leves a graves, y 3 de ellos fallecieron. En la infección por SARS-CoV-2 se alteran diferentes rutas inflamatorias, en las que la colchicina reduce los niveles de citocinas y la activación de macrófagos, neutrófilos e inflamasoma. Revisamos también, en este artículo, los posibles mecanismos que puede utilizar colchicina para prevenir el síndrome de distrés respiratorio agudo (SDRA) en pacientes con COVID-19.
Coronavirus (COVID-19) infection and the mortality associated with the acute respiratory distress syndrome (hereafter referred to as ARDS) poses a global public health challenge.1 To date, it exceeds two and a half million infections worldwide with a mortality rate that is greater than 7.08%. The increase in the spread and associated mortality poses a scenario where cost-effective therapeutic options to control the epidemic and decrease the number of deaths are urgently recommended to the international scientific community. Several reports show that the common final event that increases mortality from COVID-19 infection is ARDS, which results from an unmodulated inflammatory response and leads to death.1,2
It has been a challenge to identify targets where drugs will useful for controlling and treating the new coronavirus COVID-19 infection. Currently several potential drugs are used at different stages of the disease; however none of the therapies have been proven to be completely effective to date.3 One possible useful drug is colchicine, a molecule commonly used to treat different diseases such as gout and some autoinflammatory syndromes such as Adult-onset Still's disease, Behçet's disease or familial Mediterranean Fever as well cardiac conditions, etc.4
Colchicine has been used for more than 10 years for symptomatic treatment of patients with iatrogenic allogenosis (IA), a disease caused by allogenic substances like modeling agents or biopolymers that are foreign to the body.5 Colchicine decreases the symptoms these patients have that are associated with inflammatory response, and some clinical manifestations such as arthralgia, headache, and pulmonary infiltrates. These patients experience clinical improvement, and the manifestations decrease in frequency.
In the light of current information, a summary of recent knowledge about colchicine mechanisms of action and metabolic pathways that might explain the immunomodulatory effects that lead to preventing ARDS associated with COVID-19 infection and its possible effects on viral replication and antigen presentation are presented.
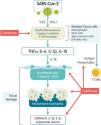
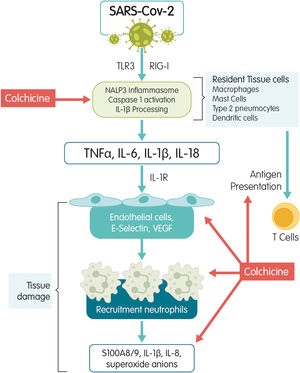
Mechanisms of actionColchicine is a tricyclic alkaloid that is extracted from the plant Colchicum autumnale. Colchicine acts as a potent inhibitor of tubulin polymerization. The mechanism studied the most is colchicine's high affinity for binding to the ß-tubulin subunit to prevent it from assembling and thus block microtubule polymerization.6 Microtubules are the key piece of the cytoskeleton and are involved in multiple cellular processes such as maintaining the shape of the cell, transferring intracellular substances, secreting cytokines and chemokines, cell migration, regulating ion channels, and cell division. Colchicine is an antimitotic substance that blocks cell division during metaphase.6 It stimulates independent GTPase activity to promote the loss of the microtubule GTP cap and prevent assembly. When colchicine binds to tubulin, the straight conformation of the aß-tubulin heterodimeric subunits is lost resulting in curved tubulin heterodimers. The lateral contacts between adjacent aß-subunits that are needed to maintain interaction between them is lost and, as lateral contacts decrease, microtubules disassemble. Colchicine may also modify the voltage-dependent anion channels of mitochondrial membranes, thereby limiting mitochondrial metabolism.7 Colchicine has an additional effect on the chemotaxis of inflammatory cells such as neutrophils and monocytes and on the intracellular transportation of vesicles such as endosomes and exosomes. It also inhibits the expression of E-selectin, an adhesion molecule important for binding leukocytes to endothelial cells and the recruitment of monocytes and neutrophils to inflamed tissue. Finally, colchicine reduces the neutrophil production of free radicals like superoxide.8 It has also been associated with disrupting inflammasome activation, thereby suppressing caspase-1 activation and the subsequent release of IL-1ß and IL-18.9 The SARS-CoV-1 infection has been associated with inflammasome activation10 by calcium ion transportation leading to IL-1-ß overproduction,11 direct caspase-1 activation, or an enhanced potassium efflux12 (Fig. 1). Within the bloodstream, ~40% of colchicine binds to albumin. Peak plasma concentrations occur 1h after administration and maximal anti-inflammatory effects develop over 24–48h based on intra-leukocyte accumulation. Colchicine reaches much higher concentrations within leukocytes than in plasma and persists there for several days after ingestion. All of this explains in part its fast blockage of acute inflammatory diseases such as gout.13
Metabolism and eliminationColchicine is eliminated from the body mainly through transportation by P-glycoprotein [P-gp, also known as multi-drug resistant protein 1 (MDR1) or ATP-binding cassette subfamily B member 1 (ABCB1)] expressed in hepatocytes (biliary excretion), proximal renal tubules (renal excretion), enteric cells (intestinal excretion), monocytes, and blood-brain barrier cells. The P glycoprotein is encoded by the MDR1 gene, and certain MDR1 polymorphisms are associated with increased P-gp expression/activity and decreased serum colchicine concentrations.14 A smaller, but significant quantity of absorbed colchicine is metabolized by hepatic P450 cytochrome CYP3A4, or directly eliminated by the kidneys through glomerular filtration. All of these mechanisms are vulnerable to drug interaction which can affect bloodstream levels.6
In general, colchicine has been safely administered. However, when there is an overdose, toxicity includes neutropenia, gastrointestinal upset, bone marrow damage, and anemia. Colchicine's toxicity is due to its antimitotic properties. It binds to tubulin in cells and causes impaired protein assembly, mitotic arrest, and multi-organ dysfunction but is safe when used according to the established therapeutic guidelines, and toxicity is rare if the recommended doses are not exceeded.7
Clinical cases description: Iatrogenic allogenosis patients infected with COVID19 under colchicine treatmentFive cases with a clinical history of biopolymers in the gluteal region who developed IA and comorbidities with a high probability of an autoimmune/inflammatory syndrome induced by adjuvants (ASIA Syndrome) or Schoenfeld's Syndrome are presented (Table 1).15 Colchicine was used for the symptomatic management of the clinical manifestations of IA. These patients were treated with an initial impregnation dose as is done in the management of an acute attack of gout: First day: 1mg every 8h. Second day: 1mg every 12h and from the third day on a maintenance dose of 0.5–1mg per day for 3–4 weeks until remission of symptoms. This is repeated between 1 and 3 times a year depending on the reappearance of symptoms such as: pain, erythema, arthralgia, headache, insomnia, and depression. In general, control is regained over the inflammatory or autoimmune process that occurred in these patients. A similar use has been shown and a favorable outcome has been achieved with colchicine in patients with foreign body granuloma as an inflammatory tissue reaction to exogenous material (therapeutic embolization of the facial arteries),16 or Granuloma faciale17 and in cases of oleoma18 (a non-allergic, foreign body type granulomatous reaction in response to oily exogenous substances injected into the dermis or subcutis for esthetical purposes).
Characteristics of patients with Colchicine treatment before COVID-19 diagnosis.
| Case | Age (years) | Weight | Colchicine dosage (oral) | Years before of biopolymers injections | Colchicine consumption before COVID-19 diagnosis | Comorbidities | Clinical manifestation | Close contact COVID-19 positive |
|---|---|---|---|---|---|---|---|---|
| 1 | 61 | 68kg | 0.5mg per day | 15 | 3 weeks | Arterial hypertension treated with enalapril 10mg/day | Subjective fever and headache during 5 days | 68-Year-old aunt who lived with her, with arterial hypertension. She died in the ICU due to ARDS and multi-system failure |
| 2 | 57 | 74kg | 1mg per day | 12 | 2 weeks before diagnosis and 1 week after | Type 2 diabetes treated with metformin | Headache during 3 days and dry cough without dyspnea | 45-Year-old sister hospitalized for dyspnea, did not require an ICU.25-year-old cousin with mild cough and headache |
| 3 | 48 | 66kg | 0.5mg per day | 8 | 1 week | Hypothyroidism treated with levothyroxine 100 mcg/day | Headache during 5 days and mild cough | Husband with headache and dry cough. Children of 14 and 19 years old were asymptomatic |
| 4 | 38 | 52kg | 0.5mg per day | 6 | 3 weeks | Fibromyalgia after biopolymers injections | Dry cough without dyspnea. Myalgias during 5 days | 56-Year-old cousin died. She does not live with her but has close contact (birthday celebration) |
| 5 | 45 | 62kg | 0.5mg per day | 10 | 2 weeks | Hashimoto's thyroiditis | Nonspecific arthralgias, subjective fever, and odynophagia | 86-Year-old grandmother who lived with her, with arterial hypertension died |
Kg: kilograms; mg: milligrams; mcg: micrograms; ARDS: acute respiratory distress syndrome; ICU: intensive care unit.
The five patients were diagnosed with IA and comorbidities (age, arterial hypertension, diabetes, hypothyroidism) and were put under colchicine treatment for IA crisis 3 weeks ago. These patients developed mild symptoms (headache, cough without dyspnea, and arthralgias) and were positive when tested for COVID-19 RT-PCRT. However, they did not need to be hospitalized for treatment. It should be noted that three close contacts presented severe symptoms and died, whereas other close contacts experienced mild symptoms. Based on these cases, we hypothesized a possible protective effect of colchicine among the IA patients who did not suffer a severe COVID-19 infection even though they had several comorbidities and a possible disrupted immune system because of ASIA Syndrome which has been reported to be a risk factor for patients with the new pandemic disease.19
Recently, Gandolfini et al.20 showed the possible colchicine therapeutic effect (applied at 120mg) on a 52-year-old female who developed COVID-19 8 months after a kidney transplant. The patient received antiviral therapy plus hydroxychloroquine. After progressive worsening of respiratory conditions requiring non-invasive ventilation and being considered for intubation, the antiviral therapy was ended, and the patient was put on colchicine (1mg on day 8, and 0.5mg/day thereafter) to reduce inflammation due to the unavailability of anti-IL-6 receptor mAb tocilizumab. Authors concluded that the immunosuppression interruption combined with the anti-inflammatory effects of colchicine may have synergized with the antiviral therapy and hydroxychloroquine to lower viral replication and minimize the cytokine storm triggered by SARS-CoV-2.
Possible uses of colchicine to prevent acute respiratory distress syndrome (ARDS) in patients with COVID-19 infectionStudies showing the behavior of the COVID-19 infection indicate that between 70 and 80% of the infected population will have mild or moderate symptoms, 15% will have severe infections manifested by severe dyspnea with hypoxemia and pulmonary infiltrates, and 5% will have critical conditions with respiratory failure, septic shock, and multi-organ dysfunction.21 Patients in higher age groups (over 60 years old) and with associated comorbidities such as hypertension and diabetes have the highest mortality rates.22 Among the predictors of worse prognosis are the presence of dyspnea, lymphopenia, elevated neutrophils, and decreased monocytes and platelets. The CD4 and CD8 T lymphocyte count is lower, and the ureic nitrogen and creatinine is higher. In other words, there is an evident suppression of cellular immunity along with a severe inflammatory reaction that can lead to death. There is a greater proportion of dyspnea symptoms in patients who die compared to survivors who do not suffer fevers, headaches, and other symptoms (P<.001).22 Other factors that are prevalent in patients who die from COVID-19 are a decrease in albumin, an increase in lactate, a slight decrease in the number of red cells and hemoglobin, and an increase in lactic dehydrogenase.23 Among the predictors of the best prognosis are a high level of lymphocytes and female gender, characteristics that appear in a greater proportion in the group of survivors.
ARDS appears to be the common final path leading to the death of the patient. All patients die of respiratory failure thus indicating that the lungs are the target organ. Furthermore, when multiorgan failure occurs, the most compromised organ after the lung is the heart followed by the kidney and liver. There is elevated procalcitonin in 0.5% of the patients, probably as an indicator of bacterial infection. Patients who die have a severe inflammatory cascade which is reflected in increased C-reactive protein and serum amyloid A.23,24
ARDS is believed to be the result of an injury to the alveolar epithelium and capillary endothelium with abnormalities in the immune system. Neutrophils are recruited to the lungs by cytokines while toxic mediators such as oxygen free radicals and proteases are activated and released.25 Extensive production of free radicals exceeds the capacity of endogenous antioxidants and causes oxidative cell damage. Factors such as endothelin-1, angiotensin-2, NF-kappa B, and phospholipase A-2 increase vascular permeability and destroy microvascular architecture thus increasing inflammation and lung damage.25 This results in an influx of protein-rich fluid into the airspaces caused by increased permeability of the alveolar-capillary barrier. Increased alveolar fluid reduces gas exchange through the alveolar-capillary membrane and results in hypoxemia and respiratory failure. However, no sensitive and specific clinical biomarkers for ARDS have been found. Dyspnea is also a predictive element.25
Efforts should focus on preventing the development of ARDS in patients with COVID-19 infections since once it occurs, mortality is very high and our health systems do not have the capacity to care for as many critically ill patients as has been seen in Italy, Spain and currently, the USA.
Therefore, we propose the use of colchicine in patients who are at risk of developing ARDS and who meet clinical and laboratory criteria that predict the appearance of ARDS.
A lot of attention has been given to the effects of colchicine on macrophages. Colchicine has been shown to modulate lipopolysaccharide-induced secretion of tumor necrosis factor (TNF) by liver macrophages in a rat model.6 In a mouse brain macrophage cell line, colchicine inhibited ATP-induced IL1ß release by preventing microtubule rearrangement and constraining activation of the Ras homolog gene family, which contains the protein kinase (ROCK) pathway. In the presence of colchicine, mouse peritoneal macrophages showed less ATP-induced permeability to ethidium bromide and less formation of reactive species of oxygen (ROS), nitric oxide (NO), IL1ß release, crystal-induced chemotactic factor, myeloid inhibitory C type-like lectin, toll-like receptors, and vascular endothelial growth factor.6 All these compounds intervene to intensify the inflammatory process and are responsible for alveolar damage, interstitial infiltration, and lung collapse. Colchicine can minimize such damage using different paths.
Colchicine has been studied as a possible treatment in other viral diseases. Taking into consideration the promising anti-inflammatory property and good bioavailability of colchicine, Lu et al.26 recently studied its role in inhibiting respiratory syncytial virus replication infection in vitro in BEAS-2B cells as well in vivo in neonatal rats. They showed that colchicine inhibited RSV infection in neonatal rats through regulation of anti-oxidative factor production. The expression of IFN-ß1 and RIG-I genes was also up-regulated in the RSV infected alveolar epithelial cells by treatment with colchicine.
An additional plausible link between the possible protective role for patients with COVID-19 and using colchicine is its effect on interferon mechanisms in viral infections that was studied decades ago.27 Reizin et al.28 demonstrated that colchicine inhibits interferon synthesis induced by the Lee strain of influenza B virus in chick embryo cells, but it does not influence the release of preformed interferon from cells. Colchicine was found to be most effective when it was introduced into the medium at early stages of infection. It may also inhibit the formation of messenger RNA for interferonogenesis.
Colchicine clinical trials in COVID19Colchicine may be a good therapeutic option for COVID-19. Currently, there are only nine clinical trials considering the clinical utility of colchicine. These include the Colchicine Coronavirus SARS-CoV2 Trial (COLCORONA) NCT04322682, the only trial recruiting patients, the ECLA PHRI COLCOVID Trial NCT04328480, the GReek Study of the Effects of Colchicine on Covid-19 Complication Prevention (GRECCO-19) NCT04326790, the Colchicine to Reduce Myocardial Injury in COVID-19 (COLHEART-19) NCT04355143, and the Colchicine Counteracting Inflammation in COVID-19 Pneumonia NCT04322565 among others that will begin recruiting patients shortly. Hopefully, their results will come out soon.
ConclusionThe global medical community does not know whether rheumatic disease or immunosuppression increases the initial risk of COVID-19 infection. It is not clear based upon current information whether the ingestion of colchicine by healthy patients may place the patient in an immunosuppressed condition.
Despite concerns that immunosuppressive drugs may increase the risk of complications for infected patients, there is hope that the anti-inflammatory properties of these drugs may mitigate lung injury. COVID-19 infection can give rise to what has been called “a cytokine storm” (i.e. macrophage activation syndrome). In such situations, there is a massive release of various cytokines. Therefore, some of the biological immunosuppressive drugs used by rheumatologists may be effective in treating serious COVID-19 infections.
For instance, interleukin blockers (IL-1 and IL-6) have been effective in ARDS. There is also evidence that tumor necrosis factor (TNF) inhibitors may be effective.
In this respect, in the risk-benefit and cost-effectiveness balance, the administration of colchicine to healthy patients or those at risk of an ARDS complication should be evaluated. The benefits exceed the risks of its use, thus, in an emergency situation caused by the current pandemic, we would recommend using colchicine.
In this context, the properties of colchicine, as previously described, can be used to decrease the risk of ARDS in patients with COVID-19 infection. Given its relatively good safety record and tolerability profile when used carefully and appropriately, it should be implemented through the proposed protocols. This would provide an opportunity to decrease the results of the infection and allow for clinical observation.
Conflict of interestsThe authors declare that there is no conflict of interests.