The association between spondyloarthritis (SpA) and inflammatory bowel disease (IBD) has been shown in many studies. More recently, with the hypothesis that increased gut inflammation is of etiopathogenic importance in the development of SpA, evaluation of anti-Saccharomyces cerevisiae antibodies (ASCA) has gained increasing relevance.
ObjectiveTo study the status and frequency of ASCA in SpA patients and the association of these biomarkers with the clinical profile.
MethodsAn observational study was performed including 231 SpA patients treated with biologic therapy. ASCA IgA and IgG levels were determined by micro-enzyme-linked immunosorbent assay.
ResultsOur data showed an increase of ASCA IgA positivity among SpA patients. No relationship was found between ASCA status and the demographic aspects, genetic factors or clinical presentation, except for the association with IBD.
ConclusionOur study confirms that ASCA IgA are elevated in SpA patients. Although there was no evidence of association with a particular disease phenotype, the existence of higher ASCA levels sustains a close relationship between gut and SpA.
La asociación entre la espondiloartritis (SpA) y la enfermedad inflamatoria intestinal (EII) se ha demostrado en muchos estudios. Recientemente, con la hipótesis de que el aumento de la inflamación intestinal es de importancia etiopatogénica en el desarrollo de la SpA, la evaluación de los anticuerpos anti-Saccharomyces cerevisiae (ASCA) ha adquirido una relevancia creciente.
ObjetivoEstudiar los niveles de los ASCA en pacientes con SpA, y la asociación de estos biomarcadores con el perfil clínico.
MétodosSe realizó un estudio observacional que incluyó a 231 pacientes con SpA tratados con terapia biológica. Los niveles de ASCA IgA e IgG se determinaron por técnica de ensayo de inmunoabsorción ligado a enzimas.
ResultadosNuestros datos mostraron un aumento de la positividad de ASCA IgA entre los pacientes con SpA. No se encontró ninguna relación entre los ASCA y los aspectos demográficos, factores genéticos o presentación clínica, excepto la asociación con la EII.
ConclusiónNuestro estudio confirma que los niveles de ASCA IgA están elevados en pacientes con SpA. Aunque no hubo evidencia de asociación con un fenotipo de enfermedad en particular, la existencia de niveles más altos de ASCA mantiene una relación estrecha entre el intestino y la SpA.
In spondyloarthritis (SpA) patients, an increased gut permeability and an asymptomatic intestinal inflammation, usually affecting the ileum and independent from the use of nonsteroidal anti-inflammatory drugs, have been reported in several studies.1,2
Anti-Saccharomyces cerevisiae antibodies (ASCA), which are rarely positive in healthy controls (<5%), possess clinical significance in inflammatory bowel disease (IBD) management.2–4 This serological biomarker is more commonly elevated in Crohn's disease (CD) than in ulcerative colitis (UC), presenting a sensitivity of approximately 72% and a specificity of 82% in CD.2–6
Because SpA and IBD share similarities and following the hypothesis that increased gut inflammation and consequent loss of mucosal immune tolerance is of etiopathogenic importance in the development of SpA, evaluation of these antibodies has gained increasing relevance.4,7
An increased prevalence of these serologic markers of IBD in SpA patients could sustain an additional pathophysiological link between the two entities.1 Furthermore, identification of new biomarkers can represent an advance for the early diagnosis of SpA as well as of important associated clinical manifestations and, consequently, this can be reflected in a significant improvement of therapeutic management.2
Although some studies have shown ASCA seropositivity to be more common among SpA patients than in healthy controls, the relationship of the antibodies status and the disease phenotype remain unclear and poorly described in literature.1,6,8 In the present study, we purpose to investigate the status and frequency of ASCA in SpA patients and the association of these serological markers with the clinical profile.
Materials and methodsPatientsWe performed an observational retrospective study including 231 SpA individuals treated with biologic therapy, followed at our Rheumatology Department, in a day hospital visit through the protocol evaluation of the National Register of Rheumatic Patients (Reuma.pt). This national database was developed by the Portuguese Society of Rheumatology and became active in June 2008, having been approved by the National Data Protection Board and local Ethics Committees. All the patients registered in reuma.pt sign an informed consent for use of the data in research projects. Patients were not required to give another informed consent to the study, since no addition samples or outpatients attendances were necessary.9 Classification of SpA was based on the Assessment of SpondyloArthritis International Society (ASAS) criteria.10
Clinical dataDemographic, clinical and immunological data were obtained by consulting the national database Reuma.pt. The following variables were collected for the analysis: gender, current age, age at diagnosis, disease duration, type of involvement (axial, peripheral or axial and peripheral), HLA-B27 status, tobacco smoking and presence or past extra-articular manifestations associated with SpA including psoriasis, inflammatory bowel disease and uveitis. ASCA IgA and IgG levels were measured in the period time between 2016 and 2017 and determined by micro-enzyme-linked immunosorbent assay (ELISA) using a commercial kit from Euroimmun, Germany. The quantitative ASCA results were expressed in RU/mL and 20 was established as the cut-off point.
Statistical analysisContinuous variables are expressed as mean (standard deviation) or median (range). Categorical variables are presented as absolute values and percentages. Chi-squared test or Fisher test were used for analysis of categorical variables and student t-test or Mann–Whitney for continuous variables. The adopted significance was of 0.05. Statistical analysis was performed using SPSS version 23.0.
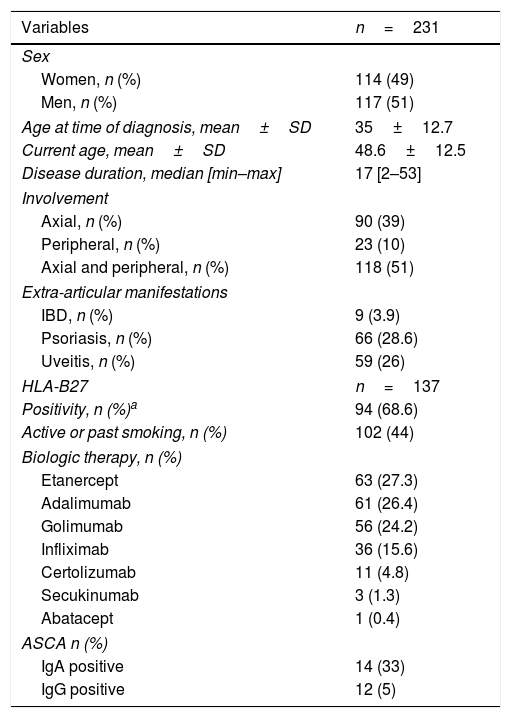
ResultsWe included a group of 231 patients with SpA, 117 of which were men (51%), with a mean age of 48.6±12.5 years. The median disease duration was 17 years [min: 2; max: 53].
In total, 39% of the patients had axial SpA (n=90), 10% peripheral SpA (n=23) and 51% presented axial and peripheral SpA (n=118). Nine patients had associated IBD (7 cases with CD and 2 with UC) and 66 patients exhibited concurrent psoriasis (28.6%). Ninety-four patients (68.6%) were HLA-B27+ and 59 (26%) presented history of uveitis (current or previous). Less than half of the patients had active or past smoking habits (n=102; 44%). The clinic and demographic characteristics are summarized in Table 1.
Demographic, clinical and immunological characteristics of the 231 SpA patients included. Abbreviations: ASCA – anti-Saccharomyces cerevisiae antibodies; IBD – inflammatory bowel disease; Ig – immunoglobulin. Superscript a: Give some missing data concerning HLA-B27 status since its presence is not mandatory for the diagnosis of SpA and taking into account that this is an observational study, the prevalence was adjusted for patients who had HLA-B27 determination.
| Variables | n=231 |
|---|---|
| Sex | |
| Women, n (%) | 114 (49) |
| Men, n (%) | 117 (51) |
| Age at time of diagnosis, mean±SD | 35±12.7 |
| Current age, mean±SD | 48.6±12.5 |
| Disease duration, median [min–max] | 17 [2–53] |
| Involvement | |
| Axial, n (%) | 90 (39) |
| Peripheral, n (%) | 23 (10) |
| Axial and peripheral, n (%) | 118 (51) |
| Extra-articular manifestations | |
| IBD, n (%) | 9 (3.9) |
| Psoriasis, n (%) | 66 (28.6) |
| Uveitis, n (%) | 59 (26) |
| HLA-B27 | n=137 |
| Positivity, n (%)a | 94 (68.6) |
| Active or past smoking, n (%) | 102 (44) |
| Biologic therapy, n (%) | |
| Etanercept | 63 (27.3) |
| Adalimumab | 61 (26.4) |
| Golimumab | 56 (24.2) |
| Infliximab | 36 (15.6) |
| Certolizumab | 11 (4.8) |
| Secukinumab | 3 (1.3) |
| Abatacept | 1 (0.4) |
| ASCA n (%) | |
| IgA positive | 14 (33) |
| IgG positive | 12 (5) |
ASCA IgA were positive in 14% of the whole sample (n=33; 14 patients with axial involvement, 4 with peripheral involvement and 15 with axial and peripheral involvement). ASCA IgG positivity was found in 5% of the SpA (n=12; 7 patients with axial involvement, 1 with peripheral involvement and 4 with both involvements). The median ASCA IgA and IgG titres were 72RU/mL [min: 22; max: 200] and 45.5RU/mL [min: 28; max: 200], respectively.
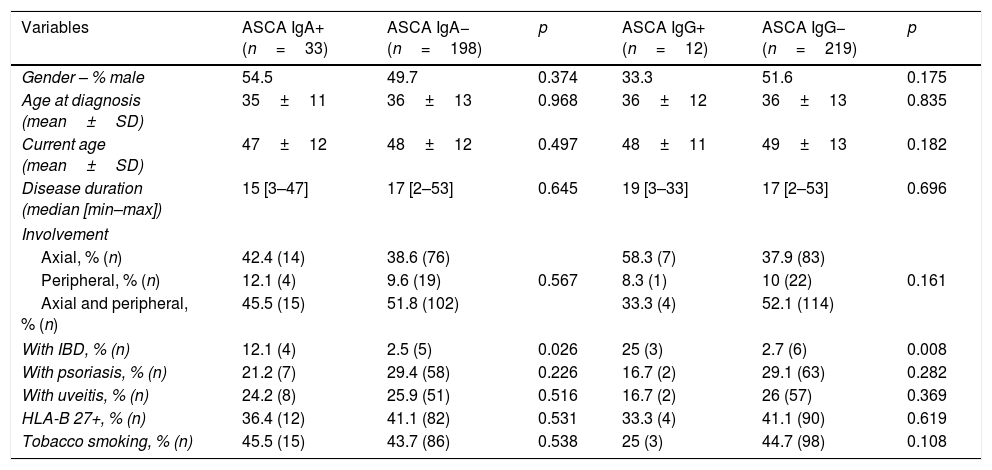
As expected, ASCA IgA positivity was associated with presence of IBD (12.1% vs 2.5%; p=0.026). We verified the same relationship with ASCA IgG positivity (25% vs 2.7%; p=0.008). Despite these results, we found no statistically significant differences in the number of ASCA IgA or IgG-positive patients according to the type of IBD-CD vs UC (p=0.722 and p=0.583, respectively).
Current age and at time of diagnosis, disease duration, gender, tobacco use were similar between ASCA IgA or IgG-positive and negative groups. Also, disease phenotype including type of involvement, presence of psoriasis or uveitis and HLA-B27 positivity were unrelated to ASCA IgA and IgG status (Table 2).
Clinical characteristics of the SpA patients according to ASCA status. Abbreviations: ASCA – anti-Saccharomyces cerevisiae antibodies; IBD – inflammatory bowel disease; SD – standard deviation.
| Variables | ASCA IgA+ (n=33) | ASCA IgA− (n=198) | p | ASCA IgG+ (n=12) | ASCA IgG− (n=219) | p |
|---|---|---|---|---|---|---|
| Gender – % male | 54.5 | 49.7 | 0.374 | 33.3 | 51.6 | 0.175 |
| Age at diagnosis (mean±SD) | 35±11 | 36±13 | 0.968 | 36±12 | 36±13 | 0.835 |
| Current age (mean±SD) | 47±12 | 48±12 | 0.497 | 48±11 | 49±13 | 0.182 |
| Disease duration (median [min–max]) | 15 [3–47] | 17 [2–53] | 0.645 | 19 [3–33] | 17 [2–53] | 0.696 |
| Involvement | ||||||
| Axial, % (n) | 42.4 (14) | 38.6 (76) | 58.3 (7) | 37.9 (83) | ||
| Peripheral, % (n) | 12.1 (4) | 9.6 (19) | 0.567 | 8.3 (1) | 10 (22) | 0.161 |
| Axial and peripheral, % (n) | 45.5 (15) | 51.8 (102) | 33.3 (4) | 52.1 (114) | ||
| With IBD, % (n) | 12.1 (4) | 2.5 (5) | 0.026 | 25 (3) | 2.7 (6) | 0.008 |
| With psoriasis, % (n) | 21.2 (7) | 29.4 (58) | 0.226 | 16.7 (2) | 29.1 (63) | 0.282 |
| With uveitis, % (n) | 24.2 (8) | 25.9 (51) | 0.516 | 16.7 (2) | 26 (57) | 0.369 |
| HLA-B 27+, % (n) | 36.4 (12) | 41.1 (82) | 0.531 | 33.3 (4) | 41.1 (90) | 0.619 |
| Tobacco smoking, % (n) | 45.5 (15) | 43.7 (86) | 0.538 | 25 (3) | 44.7 (98) | 0.108 |
Our results showed that SpA patients present an increase of ASCA IgA positivity, in agreement to previous data. Of interest, we also noticed that this evidence is mainly directed to the IgA subclass and no to IgG antibodies, as observed in others reports.1,2,4,5,7
Although the raise of ASCA in SpA patients, the data regarding the role of ASCA in the clinical profile and prognostic disease of Spa patients remain discordant.
In 2016, Maillet et al. found that the presence of ASCA was associated with a particular SpA phenotype characterized by the absence of axial involvement, presence of IBD, arthritis and past or present history of uveitis.11
In contrast, Hoffman et al. did not find differences in ASCA IgA levels comparing patients with or without peripheral synovitis.4 Also, in the study of Marianne et al. no association of ASCA IgA could be demonstrated with the occurrence of peripheral arthritis or uveitis or with the presence of HLA-B27.7
In our study, we did not find any relationship of ASCA status and the demographic aspects, genetic factors or clinical presentation, except the association with presence of IBD. However, despite the differences were not statistically significant, a higher number of patients with ASCA IgA and IgG positivity was found in whom axial involvement was present.
The data published in 2014 by Praet et al. support that gut inflammation is linked to progressive disease in axial SpA through the evidence of higher degrees of bone marrow oedema in sacroiliac joints, assessed by the Spondyloarthritis Research Consortium of Canada scoring system, in SpA patients with histological findings of chronic mucosal lesions.12
Also, according to Aydin et al., patients with positivity to ASCA had greater severity of spine radiological damage evaluated by the Bath Ankylosing Spondylitis Radiology Index, suggesting its potential role as a marker of structural disease progression.5
Some theories point that an increased intestinal permeability in SpA, as shown by ASCA positivity, can be related to a higher radiological damage as a result of ongoing antigenic stimulus.5 The present study was not designed to evaluate the impact of ASCA status in the structural and radiological damage, but further prospective studies are warranted to clarify the previously speculated hypothesis.
A limitation of the current study is that all the SpA patients included were under biologic therapy, which might raise issues about a possible influence on antibody reactivity. Nevertheless, it has been demonstrated that antibody responses do not change over time or as a result of drug therapy, including biologic therapy.13
In the future, more studies would be necessary to investigate the relationship between antibody reactivity and endoscopic findings in SpA patients. Additionally, it would be interesting to assess the faecal calprotectin levels, since it is a recognized standard clinical biomarker of IBD activity and recently, as suggested by Moreno Martínez et al. a parameter elevated in SpA patients which correlates positively with C-reactive protein.8,14 Finally, a longer follow-up of these patients, assessing the onset of new gastrointestinal symptoms and the subsequent development of IBD, will be critical in determining if the presence of ASCA is a predictive marker of the SpA phenotype.
Conflict of interestNone declared.