Rheumatoid arthritis (RA) is a systemic inflammatory disease affecting the synovium of joints, tendons, and some extra-articular sites. RA prevalence in Latin America ranges from 0.4 to 1.6%. Early treatment of RA translates into a substantial reduction in the cost to society. In light of this, early disease clinics are being established in some countries. Barriers to RA management, such as delay in referral to rheumatologists and limited access to therapy, have been identified. Evidence-based treatment guidelines have been adapted by countries according to their own situations. The need for keeping accurate records of biologics prescribed has been addressed by biologic registries, thereby contributing toward a better understanding of rheumatic diseases and their treatment. Current biologics include the tumor necrosis factor (TNF)-α inhibitors (etanercept, infliximab, and adalimumab), B-cell depletion agent (rituximab), interleukin-6 receptor blocker (tocilizumab), and T-cell co-stimulatory blocker (abatacept). Future therapies include kinase inhibitors (tofacitinib and fostamatinib), alternative TNF-α inhibitors (golimumab and certolizumab), and biosimilars.
La artritis reumatoide (AR) es una enfermedad sistémica e inflamatoria que afecta la membrana sinovial de las articulaciones, los tendones y algunos sitios extra-articulares. La prevalencia de la AR en Latinoamérica se encuentra entre 0.4–1.6%. El tratamiento precoz de la enfermedad se traduce en una reducción del costo para la sociedad. En vista de esto, se han establecido clínicas de AR temprana en varios países de la región. Se han identificado barreras para el tratamiento de la AR como lo son el retraso en la referencia al reumatólogo y limitaciones en el acceso al tratamiento. Varios países han desarrollado y adaptado guías para el tratamiento basadas en la evidencia y en sus propias realidades. La necesidad de tener registros detallados de las prescripciones de biológicos ha sido abordada con registros de biológicos lo que llevará a un mejor entendimiento de las enfermedades reumáticas y su tratamiento. Los biológicos disponibles en la actualidad son los inhibidores del factor de necrosis tumoral (TNF)-α (etanercept, infliximab y adalimumab), un agente depletor de células B (rituximab), un bloqueador del receptor de interleucina-6 (tocilizumab) y un bloqueador de la co-estimulación de células T (abatacept). En el futuro se incluirán los inhibidores de cinasas (tofacitinib y fostamatinib) e inhibidores del TNF-α alternativos (golimumab y certolizumab) y biosimilares.
Rheumatoid arthritis (RA) is clinically recognized as an inflammatory process that mainly affects the synovium of joints and tendons and often some extra-articular sites. Classically, the criteria for RA classification relied on the presence of signs and symptoms indicative of inflammatory activity which typically implied irreversible structural damage when present. This situation led to the addition of a new set of classification criteria endorsed by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) for the diagnosis of RA. The aims of such an initiative were to establish a set of rules to be applied to newly presenting patients with undifferentiated synovitis that would: (1) identify the subset at high risk of chronicity and erosive damage; (2) be used as a basis for initiating disease-modifying therapy; and (3) not exclude the capture of patients later in the disease course.1 In this way, early and appropriate disease-modifying treatment, aimed to reduce the risk of joint destruction, could be initiated.
At first glance, early recognition of patients at risk of erosive RA and swift initiation of treatment should be achievable worldwide. Programs for early diagnosis of patients with undifferentiated arthritis at risk of RA can be instituted at the level of primary health clinics. Once diagnosed, at-risk patients can be treated with disease-modifying antirheumatic drugs (DMARDs) with or without biologic agents with the goal of achieving disease remission and avoiding structural damage. While these goals and strategies have already been launched in the European Union, Canada, and the United States, less progress has been achieved in Latin America. The success of these programs across the world depends on who covers the cost of health care. In Europe and Canada, where health care costs are covered by the state, coverage for RA, for example, depends on the percentage of the health-care budget allocated to rheumatic diseases. In Latin America, the funding situation differs among countries where many state health budgets are limited. Consequently only a fraction of the population has full health coverage, and many patients pay for expenses out of pocket to cover the cost of medical care and insurance. In this sense, the implementation of programs for early detection and treatment of RA might be less successful than in developed countries.
The objectives of this paper are to examine existing treatment practices for RA and discuss the socioeconomic impact of RA from a Latin American perspective.
EpidemiologyThere are few reports on the prevalence of RA in Latin America. Recent reports from Argentina show prevalence rates ranging between 0.2% in Tucuman province and the city of Buenos Aires and 0.94% in Lujan province in the province of Buenos Aires, Argentina.2–4 The recent epidemiological study on musculoskeletal pain involving 25,587 subjects conducted in five regions in Mexico (Chihuahua, Nuevo Leon, Sinaloa, Yucatan, and Mexico City) showed an overall RA prevalence of 1.6% (95% CI: 1.4–1.8)5; the prevalence in females was 2.0%, while that of males reached 0.8%. The prevalence of RA varied across country regions, specifically, from 0.7 in the Nuevo León state to 2.8% in Yucatán state. One previous study in México City showed a prevalence of 0.4%.6
Latin America, with a heterogeneous population estimated at 577 million people, has young adults as the dominant demographic group. RA can start at an early age, as shown by a study comparing patients from Mexico with those from Canada. Mexican patients developed RA almost 12 years earlier than Canadians (95% CI: 9–15 years, P<.0001).7 Almost half of Mexicans, compared with a quarter of Canadians, had their first swollen joint before the age of 36 years. About 4% of Mexicans and 35.5% of Canadians had onset of RA after the age of 55 years (P<.001). RA mainly affects women in their productive years of life.
Socioeconomic impact of rheumatoid arthritisLatin America, like many other developing regions of the world, has undergone an epidemiological shift from acute to chronic disease as the major contributor to morbidity and mortality, while still confronting problems such as poverty and malnutrition.8 Consequently, these changes impact the allocation of health-care resources from acute diseases, such as infections, to chronic diseases such as RA.9 RA is a chronic disease with substantial burden to the patient (reduced function and lower quality of life), society (loss of productivity through sick leave or permanent work disability [PWD]), and on health-care resources. 10 In Mexico, for example, the annual cost of RA and ankylosing spondylitis corresponded to $2900 and $2800 USD, respectively, when direct costs and out-of-the-pocket expenses are considered.11 More recently, we estimated the annual cost of RA per patient in $5534 USD dollars including indirect costs.12 The reported prevalence of PWD in the country due to RA is 11.7% and this represents a significant cost to Mexican society and to the Instituto Mexicano del Seguro Social (IMSS), which is the main health-care provider for salaried workers in the country.13 A lower education level, treatment delay, and positive rheumatoid factor (RF) were shown to increase the risk for PWD in Mexicans with RA.
Health care is typically fragmentary in Latin America. In some countries, the state provides for the majority of health care while in others, individuals pay all or some of the medical expenses. Reimbursement is very limited and is only available in some countries. Access programs are available in some countries where the government usually provides the drug. The coverage varies from 60 to 100% of the population and not all biologics are available. Mexico has the social security or institutional programs of Petróleos Mexicanos (PEMEX) and Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, Brazil has Sistema Unico de Saude, Venezuela has the Instituto Venezolano de los Seguros Sociales which offers almost 100% coverage, Colombia has the Entidades Promotoras de Salud, Ecuador has partial and very limited coverage through social security, and Peru has very limited coverage by social security or by other government institutions. Argentina has different governmental and private programs offering different degrees of coverage. In Chile, the public health-care system accounts for 80% of the population while the remaining 20% is covered by private health care.
Early treatment of RA translates into substantial reduction in the cost to society.14 In light of this, early disease clinics are being established in some countries, including Argentina, Colombia, Brazil, Mexico, and Peru. A Colombian study showed that the time to referral from primary to tertiary care for RA patients improved from 1998 to 2002 with the introduction of early disease clinics; 54% of patients were referred within 3–6 months of diagnosis by 2002 and DMARDs were shown to be the leading first-line and second-line treatment options, with biologics accounting for 16% of second-line prescriptions in 2002.15 In Argentina, the Consorcio Argentino de Artritis Temprana database was established that specifically focuses on patients with early RA (ERA) to provide further data on detection and appropriate follow-up. Preliminary results showed a frank reduction in disease duration at referral in less than two years.16
Barriers identified in the management of RA include inadequate health-care access and referral delays due to the lack of training of general practitioners in musculoskeletal problems, a shortage and inadequate distribution of rheumatologists in some countries, and limited access to appropriate therapy. There is also a lack of patient databases or registries for rheumatic patients in most of the Latin American countries. Additional factors contributing to poor patient prognosis include low socioeconomic status, disease related factors (e.g., severity, joint damage, comorbidities), and cost of treatment. In one study, it was reported that 15% of the family income goes to RA-related expenses and this represents 26.1% of the total annual cost per RA patient.11 In a study of 262 patients with RA in Mexico, the RA annual cost reported was USD 5534.80 per patient; 65% were direct costs (physician consultations, hospitalization, medication, and alternative therapies as well as laboratory and auxiliary tests, transportation and meals related to medical care) and 35% were indirect costs (work disability, home care, and loss of income at home),12 which are similar in distribution to what has been reported in Europe.17 RA out of pocket costs caused catastrophic expenses in 46.9% of households which were associated with the type of health-care coverage (OR=2.7, 95% CI: 1.6–4.7) and disease duration (OR=1.024, 95% CI: 1.002–1.046).12 Impoverishment, measured by the household inability to cover the basic food basket threshold in Mexico, occurred in 66.8% of households and was associated with catastrophic expenses (OR=3.6, 95% CI: 1.04–14.1), high health assessment questionnaire (HAQ) scores and low socioeconomic levels.12 An analysis utilizing the Quantitative Standard Monitoring of Patients with RA database cohort of 6004 patients from 25 countries evaluated the disparities of RA disease activity with gross domestic product (GDP) of a country. It found the burden of RA was substantially greater in low GDP than in high GDP countries.18 In Latin America (which has a significantly lower GDP than Europe, the United States, or Canada), the cost of biologics in general is very similar to countries with higher GDP, further impairing access to these medications.
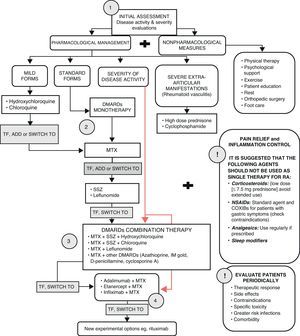
Latin American clinical criteria for the use and selection of biologics and treatment strategiesIn 2006, the Latin American Rheumatology Associations of the Pan-American League of Associations for Rheumatology (PANLAR) and the Grupo Latino Americano de Estudio de Artritis Reumatoide (GLADAR) issued the first Latin American position paper on the pharmacological treatment of RA that advocated for early aggressive therapy for RA patients.19 Efforts were focused on the issues facing the region with regards to the availability of appropriate treatment for RA and the development of treatment guidelines (shown in Fig. 1) that can be used for clinical practice.
RA treatment. TF (maximum doses after 8–12 weeks; DAS28>3.2).19
Subsequently, PANLAR and GLADAR issued a consensus position paper on the management of patients with RA in Latin America in 2009,20 establishing the following criteria for using biologic agents in RA patients who were unresponsive to DMARDs:
- •
Patients having the diagnosis of RA according to ACR criteria.
- •
Active disease measured by a disease activity scale such as disease activity score in 28 joints (DAS28) ≥3.2 and after at least 3 months on conventional treatment with DMARDS.
- •
Patients should have used two DMARDs, one being methotrexate (MTX) in adequate doses, unless there were significant adverse effects.
- •
Adequate doses of MTX should have been up to 25mg weekly via oral, intramuscular, or subcutaneous (SC), and leflunomide 20mg per day; with the exception of patients presenting adverse events (AEs) limiting the use of these therapies.
- •
Patients should be in functional ACR status I, II, or III.
GLADAR also developed evidence-based guidelines specific for the use of rituximab in the treatment of RA in Latin America.21 GLADAR recommends rituximab in patients with active (DAS28>3.2) RF-positive RA who had inadequate response or intolerance to an adequate course as previously defined with tumor necrosis factor (TNF) inhibitors. Rituximab can also be used in patients who have inadequate response or intolerance to more than one conventional DMARD and cannot receive TNF inhibitors (due to contraindications or unavailability). Based on lack of strong evidence, rituximab cannot be recommended to RF-negative RA patients; GLADAR, however, recommends that RF-negative patients be considered for treatment if they meet the conventional treatment failure criteria.21
Some countries have developed their own treatment guidelines for RA based on the PANLAR/GLADAR guidelines but adapted to their own situations. In Argentina, clinical practice guidelines for RA were developed by the Argentine Society of Rheumatology in 2003 and were updated in 2008. It has since undergone annual updates to keep up with the continuing advances in the understanding of the pathogenesis, evolution, and treatment of RA to include new diagnostic technologies and new drugs.22 In Brazil, the Brazilian Society of Rheumatology (SBR) published the “Brazilian Consensus for the Diagnosis and Treatment of RA” in the 2004 Brazilian Journal of Rheumatology. The consensus was updated in 2007 to include grades of recommendations and strength of evidence based on the previous work of SBR, the experiences of rheumatologists, and literature review.23 In Chile, the Ministry of Health issued the RA Clinical Guide Series in 2007 which included flow diagrams in the management of patients with RA and early diagnosis, referral, and treatment among its key recommendations.24 In Colombia, the Colombian Association of Rheumatology published the first edition of the “Guidelines for the Treatment of RA” in 2002. The guidelines were intended to minimize clinical variability of managing RA and to facilitate the clinical evaluation, financial, and legal processes, as well as streamlining cost associated with RA treatment.25 In Costa Rica, the Costa Rican Association of Rheumatology and the College of Physicians and Surgeons developed the “Guidelines for the Management of RA” 2010 Consensus, which considered the severity of RA as a disease that can eventually be modified and the need for close monitoring of the patient preferably by a rheumatologist.26 In Mexico, the Mexican College of Rheumatology issued guidelines and recommendations in 200627 and the IMSS published guidelines in 2010 for the treatment of RA recommending biologics.28
Current clinical practiceLatin American rheumatologists are aware of the importance of early detection and diagnosis of RA and the short- and long-term efficacy of DMARD or DMARD plus biologic agents. However, the implementation of early detection clinics and early treatment can be difficult because of inadequate financial resources needed to fund such programs. One of several strategies to minimize cost of early detection and treatment would be combining the presumptive diagnosis of RA by a rheumatologist with the presence of anti-cyclic citrullinated peptides (CCP) antibodies. Such combination was shown to be highly specific for diagnosing RA in patients with very early arthritis. The sensitivity and specificity of prediction by a rheumatologist were 94% and 74%, respectively; for anti-CCP antibodies, they were 56% and 96%, respectively. Combining the two variables resulted in a specificity of 100% and a sensitivity of 53%, and a positive predictive value of 98%.29
While debate exists about when is the best time to start treatment (within 3, 6, or 12 months), the aim is to treat as early as possible, according to patient needs. Treat-to-target with the objective of disease remission and/or low disease activity (LDA) score is practiced in some clinics in the region; close monitoring of AEs and consideration of patient comorbidities are necessary. It is important to consider each patient individually and to adopt practical strategies to meet their needs. Tight control strategies versus “routine” practice has been assessed in studies, suggesting that intensive patient management and treating to target reduce disease activity and radiographic progression compared with routine or symptomatic outpatient care.30,31
Very early rheumatoid arthritis/early rheumatoid arthritisExpert consensus defined very early RA as the RA occurring between 3 and 4–6 months and early RA (ERA at one year). Early diagnosis of RA can be difficult because patients who have very recent onset arthritis (≤12 weeks of evolution) may have been diagnosed with other diseases, including self-limited arthritis. Hence, the sensitivity and specificity of classification criteria for RA established by the ACR are not effective for this particular group of RA patients.29 Further, 80.4% of early inflammatory arthritis was shown to progress to persistent arthritis.32
For the management of early arthritis or ERA, the EULAR task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) developed a set of 12 recommendations based on evidence in the literature and expert consensus; detailed descriptions are published elsewhere.33
Biologic registriesEducating the physicians, patients, community, and policy makers is important in RA treatment. Equally important is maintaining accurate records on all aspects of prescribed biologics, particularly on AEs and treatment duration. The establishment of local disease registries addresses this need and provides a better understanding of rheumatic disease and its treatment in the region. To address safety concerns, the Pan-American Registry of AEs of Biological Therapies in Rheumatic Diseases was established in 2007 as a collaboration between PANLAR and the Sociedad Española de Reumatología (SER). It is considered an important resource to monitor biologic-related AEs in the Latin American region. This registry includes data on 1481 rheumatic-disease patients available from 1998 to 2009.34 Some country-specific biologics registries include BIOBADASAR (Argentina), established in 2010, BIOBADABRASIL (Brazil) and BIOBADAMEX (Mexico), both established in 2008. The implementation and maintenance of these registries require substantial funding and monitoring. Although initially established by national societies of rheumatology, registries also receive financial support from pharmaceutical manufacturers (e.g., BIOBADABRASIL) and essential academic support from SER.
Prescribing biologics in Latin AmericaRheumatologists in Latin America have clinical experience in treating RA patients who have access with TNF inhibitors and other biologics. Patients were treated with biologics after experiencing MTX failure, multiple DMARD failures and experiencing early, aggressive RA who are both MTX and biologic naïve following regional guidelines. A list of the biologic agents for the treatment of RA in Latin America is presented in Table 1.
Biologic agents for the treatment of RA in Latin America.
| Mechanism of action | Biologic agents |
| TNF-α inhibitors | Etanercept,35 infliximab,36 adalimumab37 |
| B-cell depletion agent | Rituximab38 |
| T-cell co-stimulatory blockers | Abatacept39 |
| Cytokine inhibitor | Tocilizumab40 |
RA, rheumatoid arthritis; TNF, tumor necrosis factor.
Kinase inhibitors currently under investigation for RA include tofacitinib (CP-690,550) and fostamatinib (R788).41,42 Tofacitinib is a janus kinase inhibitor shown in phase 3 trials to be effective in patients with moderate to severe active RA; ACR50 response rates at month 6 of up to 37% in tofacitinib versus 12% in placebo patients who had inadequate response to MTX were achieved with improvement in pain and physical function compared with placebo.43 Fostamatinib (R788) is a spleen tyrosine kinase inhibitor that was evaluated in a 6-month, double-blind, placebo controlled trial in patients with active RA and on long-term MTX therapy. Results showed ACR50 response rates of up to 43% in fostamatinib patients versus 19% in placebo patients.44 Other biologic agents for future consideration in Latin America include the monoclonal antibodies golimumab and certolizumab pegol. Golimumab was shown in a randomized, double-blind, placebo-controlled phase 3 trial to reduce the signs and symptoms of RA in patients who received prior TNF-inhibitor therapy; ACR20 was achieved at week 14 by 35% and 38% of patients on 50mg and 100mg, respectively.45 Certolizumab pegol plus MTX was shown in a randomized, double-blind, placebo-controlled phase 3 trial to be more efficacious than placebo in patients with active RA who achieved an ACR20 response.46 Certolizumab pegol is expected to be approved in Chile and Mexico in 2012; its approval is pending in Argentina and Ecuador.
BiosimilarsBiosimilars are biopharmaceuticals that may be similar to the innovator product and enter the market when the patent of the original biologic product expires (Table 2). Although the development of biosimilar or “follow on” proteins is expensive, an estimated 30% of costs can be saved on current patented biologics.47 While biosimilars are comparable to their biologic counterparts in safety and efficacy,48 they are not identical due to the manufacturing process, which is more complex than that of generic small molecule products.47 Minor changes present in biosimilars could be immunogenic, thus possibly interfering with efficacy and safety. As such, efficacy and safety data of biologics should not be extrapolated to biosimilars; neither should biologics and biosimilars be considered interchangeable unless strict biochemical and clinical studies with long term evaluation are performed.49 Furthermore long term safety of either agent cannot be adequately assessed if patients change a biologic for a biosimilar or vice versa. Pharmacovigilance is particularly important with the use of any biologic.50 Biosimilars should be considered interchangeable with the original product only after sufficient clinical data and marketplace experience have been accrued. Approval of biosimilars should be tightly regulated and require direct head-to-head toxicological, nonclinical, and clinical data in comparison to the reference biologic. Regulatory aspects are particularly important, and clinicians should be included in the review process.51
Biosimilar agents for the treatment of RA.
| Biosimilar/company | Current status | Original product (biologic) |
| TL011/Teva Pharmaceutical Industries Ltd.54 | Phase 1 and 2 clinical trials | Rituxan (rituximab) |
| Reditux/Dr Reddy's Laboratories53 | Launched in 2007 and is marketed in India and several other countries | Rituxan (rituximab) |
| Yisaipu/Shanghai CP Guojian Pharmaceuticals Co., Ltd.55 | In use in China since 2005 | Enbrel (etanercept) |
| Etanar/Lafrancol52 | In use in Colombia | Enbrel (etanercept) |
| PRX-106/Protalix Biotherapeutics56 | Preclinical | Enbrel (etanercept) |
| TNFcept/LG Life Sciences57 | Phase 1 | Enbrel (etanercept) |
| TNFmab/LG Life Sciences57 | Preclinical | Remicade (infliximab) |
RA, rheumatoid arthritis.
Colombia in Latin America had approved the etanercept biosimilar, Etanar, for the treatment of inflammatory diseases, although regulations for the approval of biosimilars are still under development. In 2006, Etanar was imported to Colombia from China, approved for the treatment of RA, and was made available through the social security system. In Colombia, the efficacy and safety of Etanar (25mg SC twice weekly) were shown in a multicenter, observational before-after study involving 110 patients with active RA despite treatment with DMARDs who were followed for 20 weeks.52 DAS28 decreased from 5.76±0.81 to 3.48±1.12 (P<.001) and HAQ decreased from 2.5±1.1 to 1.1±0.9 (P<.001). Side effects were reported in 10% of patients. Reasons for discontinuation were lack of efficacy, heart failure, nausea, dizziness, pneumonia, and asthma.52
Recently Reditux, a rituximab biosimilar produced by Dr Reddy's Laboratories (India) has become available in some countries of Latin America including Peru, Ecuador, Chile, Bolivia, and Venezuela.53
ConclusionsIn summary, it is clear that the policy of most Latin American academic rheumatologists is to diagnose as early as possible, treat early with conventional DMARDs alone or in combination, aim at remission or LDA (treat-to-target) and then add biologics if disease control is not adequate.
Management of RA has a defined treatment pattern that is adjusted based on patients’ response to therapy. Typically, MTX is the initial therapy and, in the case of inadequate response or adverse reaction, is followed by adding in combinations of other DMARDs. This is then followed by biologics, which are either cycled, in the case of TNF-α inhibitors, or changed based on their mechanism of action. The concept of early treatment and treat-to-target are well recognized in academic rheumatology but still need to be established in general practitioners’ minds and payers.
Challenges for Latin American countries, as in other parts of the world, in the treatment of RA include making RA a public health priority, knowing its socioeconomic impact in terms of its high cost and burden on the health-care system, and increasing access to prompt diagnosis, treatment by rheumatologists, and availability of effective low cost medications. Other issues of concern include regularly updating guidelines, developing treatment algorithms based on local needs and resources, establishing routine epidemiological surveillance, and educating the people, patients, and health-care providers.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestRubén Burgos Vargas has participated on advisory boards for Abbott, BMS, Jannsen, Pfizer, and Roche, and as a speaker for Abbott, BMS, Jannsen, Pfizer, and Roche.
Kasmir Ostojich was a Regional Medical Director of Medical Affairs for Pfizer Inc, Inflammation during the development of this manuscript.
Mario H. Cardiel is a clinical researcher who has conducted clinical trials for Pfizer, Roche, Bristol Myers Squibb, La Jolla Pharmaceutical, Anthera, Astra Zeneca, Chelsea. He has been a speaker and consultant for Pfizer, Bristol Myers Squibb, Abbott and Merck.
Editorial/medical writing support was provided by WC Hatch at ACUMED and was funded by Pfizer Inc. No financial support was provided to the authors for this work.