To investigate peripheral enthesitis with power Doppler ultrasound (PDUS) in patients presenting low back pain (LBP) and metabolic syndrome (MetS) in comparison with patients with only LBP, to correlate US scores with clinical-anthropometric characteristics, and to define any relationship between enthesitis and concurrent diffuse idiopathic hyperostosis syndrome (DISH).
MethodsSixty outpatients with LBP and MetS, evaluated with multi-site entheseal PDUS, scoring inflammatory and structural damage changes, were retrospectively analyzed. A group of 60 subjects with LBP, without MetS and evaluated with the same protocol, was analyzed as the control group.
ResultsPatients showed overweight (BMI 29.8) and low-grade inflammatory state (C-reactive protein [CRP] 0.58mg/dL, erythrosedimentation rate [ESR] 20.2mm/h). Enthesitis was demonstrated in 52 (86%) patients (17.6% entheses), and in 8 controls (13.3%) (p<.00001). PD signals (15% of patients) were associated with entheseal pain (p=.0138). US scores correlated with body mass index (BMI), pain, type 2 diabetes. In 28 (46%) patients a concurrent DISH was diagnosed, correlating with older age (p<.0001), CRP (p=.0428), ESR (p=.0069) and PDUS scores (p=.0312 inflammatory, p=.0071 structural). MetS had a strong association (OR 4.375, p=.0007) with concurrent DISH.
ConclusionsDiffuse peripheral enthesitis is very common in MetS. Almost half of MetS patients can have a concurrent diagnosis of DISH; they are older, with higher inflammation, and higher PDUS enthesitis scores.
Estudiar la entesitis periférica con ecografía Power Doppler (PDUS) en pacientes que presentan dolor lumbar (DL) y síndrome metabólico (SM), en comparación con los pacientes con DL únicamente, para correlacionar las puntuaciones ecográficas con las características clínico-antroprométicas, y definir cualquier relación entre entesitis y síndrome de hiperostosis idiopática difusa (DISH).
MétodosSe evaluaron 60 pacientes con DL y SM mediante PDUS para entesitis de múltiples sitios, valorando retrospectivamente los cambios inflamatorios y de daños estructurales. Un grupo de 60 sujetos con DL y sin SM fue evaluado utilizando el mismo protocolo, como grupo control.
ResultadosLos pacientes reflejaron sobrepeso (IMC 29,8) y estado inflamatorio de bajo grado (proteína C reactiva [PCR] 0,58 mg/dL, tasa de eritrosedimentación [TES] 20,2 mm/h). Se demostró entesitis en 52 (86%) pacientes (17,6% entesis), y ocho controles (13,3%) (p < 0,00001). Las señales PD (15% de pacientes) estuvieron asociadas a dolor debido a entesitis (p = 0,0138). Las puntuaciones ecográficas se correlacionaron con índice de masa corporal (IMC), dolor y diabetes tipo 2. En 28 (46%) pacientes se diagnosticó DISH concurrente, correlacionado con edad avanzada (p < 0,0001), PCR (p = 0,0428), TES (p = 0,0069) y puntuaciones PDUS (p = 0,0312 inflamatoria, p = 0,0071 estructural). El SM tuvo una fuerte asociación (OR 4,375, p = 0,0007) con DISH concurrente.
ConclusionesLa entesitis periférica difusa es muy común en el SM. Casi la mitad de los pacientes con SM pueden tener diagnóstico concurrente de DISH; son pacientes de edad avanzada, con mayor inflamación, y mayores puntuaciones de entesitis mediante PDUS.
Metabolic syndrome (MetS) is a clinical condition characterized by central obesity (measured as waist circumference ≥94cm in europids men and ≥80cm in women) associated to any two of four additional factors such raised triglycerides level (≥150mg/dl), reduced high-density lipoprotein-cholesterol (<40mg/dl in males and <50mg/dl in females, or specific treatment for these lipid abnormalities), raised blood pressure (systolic blood pressure≥130 or diastolic blood pressure≥85mmHg, or treatment of previously diagnosed hypertension), raised fasting plasma glucose (≥100mg/dl, or previously diagnosed type 2 diabetes).1 Primary management for the MetS is healthy lifestyle promotion, that includes caloric restriction to achieve loss of body weight, change dietary composition to reduce saturated fat, and increases in physical activity. In the other hand, dysmetabolic overweight patients have a high prevalence of musculoskeletal complaints which could negatively affect quality of life and impair their therapeutic physical activities.2,3
Many studies have been focused to the association of MetS with the cardiovascular risk and the development of type 2 diabetes, demonstrating a correlation of MetS with systemic inflammation, while fewer observations describe the association of MetS with musculoskeletal conditions, as isolated tendinopathies and diffuse idiopathic hyperostosis syndrome (DISH) or Forestier's disease, a senile condition characterized by ossification and calcification of peripheral and spinal entheses.4–7
Entheses are defined as the site of attachment of tendons, ligaments or joint capsules into the bone. The prevalent inflammatory involvement of enthesis is defined enthesitis and it is notoriously considered a hallmark of spondyloarthritis (SpA), while degenerative involvement of enthesis (enthesopathy) has been associated to not-inflammatory conditions as overuse, biomechanichal disturbances and DISH.8,9
Musculoskeletal power Doppler ultrasound (PDUS) has been extensively applied to the study of entheses.10 PDUS has been recently used also in dysmetabolic patients, with unexpected high prevalences of both inflammatory and degenerative changes of these structures, but the real prevalence and characteristics of entheseal involvement in MetS has yet to be clarified.3,7,11
The aim of our work was to study the PDUS-defined entheseal changes in MetS and in controls, to correlates the PDUS enthesitis scores to clinical characteristics of dysmetabolic patients and to define if there was any relation between MetS-related enthesitis and the presence of concurrent DISH.
Patients and methodsA group of 60 consecutive outpatients (24 males, 36 females, mean age 60.2 years) referred in our secondary care setting for low back pain (LBP), and all fullfilling International Diabetes Foundation (IDF) criteria for metabolic syndrome,1 were retrospectively computed.
All the patients were also evaluated with routine multi-site bilateral PDUS entheseal examination (shoulders, elbows, hips, knees and heels) by an expert rheumatologist sonographers. Each patient underwent bilateral dynamic B-mode and PDUS examination of twelve entheseal sites: acromial deltoid insertion, supraspinatus, lateral and medial elbow epicondyles, triceps, greater trochanteric enthesis, quadriceps and patellar tendon, ileo-tibial tract, Achilles tendon and plantar fascia. PDUS was performed using Esaote MyLab Twice machine equipped with 6–18MHz transducer and standardized B-mode and Doppler settings, which were optimized for all examinations. Doppler parameters were pulse repetition frequency within 500–750Hz and Doppler frequency within 7–11.1MHz.
Each enthesis was studied as the 2mm zone of soft tissue adjacent to the bone cortex, based on OMERACT's definition.12 Enthesitis was defined, for each site, when only mandatory inflammatory lesions (enthesis thickening and hypoechogenicity) were present. Power Doppler intra-entheseal signal was recorded as sign of active enthesitis. Structural abnormalities (erosions, enthesopytosis/calcification) were also recorded.
Inflammatory and structural changes were scored as a whole when present (score 1) or absent (score 0). The sum of entheses with inflammatory and structural damage was recorded and defined as “global inflammatory score” and “global structural damage score” for each patient. The prevalences of enthesitis in dysmetabolic patients were thus calculated, and global scores were also correlated with some characteristics of the group study: body mass index (BMI), presence of type II diabetes, erytrosedimentation rate (ESR), C-reactive protein (CRP), sex and age.
The Leeds Enthesitis Index (LEI) was also applied in each patients.13 Spinal radiography was available for each patient to research for signs of DISH. A diagnosis of DISH was made when Resnick and Niwayama criteria were satisfied on spinal radiography14: presence of bony bridges in anterolateral spine location, and in at least 4 contiguous vertebral bodies, with relatively preserved intervertebral spaces.
Sixty consecutive subjects, referred in our secondary care setting for low back pain (LBP), with comparable age and sex, normal weight (BMI<25kg/m2) and/or normal waist circumference, and absence of criteria for MetS (except for hypertension) were computed as controls, as they were evaluated with the same clinical routine protocol.
Exclusion criteria for both groups were: suspected inflammatory arthropathies and presence or familiarity for psoriasis. The presence of eventually concurrent not-inflammatory musculoskeletal complaints was not considered an exclusion criteria.
All the patients gave written informed consent to underwent the cited routine clinical practice. The research was carried out in compliance with the Helsinki Declaration, but no ethical committee approval was necessary in consideration of retrospective modality of acquiring the data.
Statistical analysisThe demographic, anthropometric, and clinical characteristics of patients and whole controls were compared. Thereafter, the entheseal US global scores found in each group were computed and compared (Table 1). Data are reported as mean±standard deviation (SD) for continuous variables, whereas categorical and dichotomous variables are reported as frequencies and percentage. The two-tailed unpaired T test was used to compare means of continuous variables between the two groups, when the distribution of data was normal, and with Welch's correction otherwise. Fisher's exact test was used to compare the percentages between the two groups for categorical variables. Multivariate and univariate linear regression analysis was applied to test the effects of variables on outcome and to define its correlation. The non-parametric Spearman rank test was applied to correlate variables. The level of statistical significance was set at a p-level of 0.05. Statistical analyses were performed using InStat GraphPad (laYolla California) statistical package.
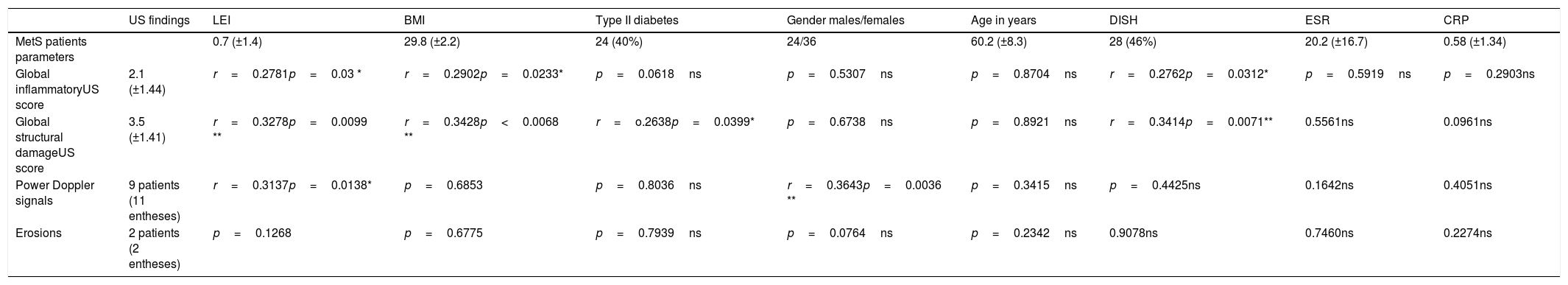
Clinical-anthropometric parameters and ultrasound findings in patients with metabolic syndrome and in controls.
| Patients with MetS | Controls | p values (statistic test) | |
|---|---|---|---|
| Number, (males/females) | 60 (24/36) | 60 (17/43) | n.s. 0.2489 (Fisher test) |
| Age, in years | 60.2 (±8.3) | 61.9 (±7.7) | n.s 0.2471 (Fisher test) |
| BMI | 29.8 (±2.2) | 23.6 (±1.1) | p<0.0001** (unpaired T-test) |
| LEI | 0.70 (±1.4) | 0.08 (±0.05) | p=0.0016** (unpaired T-test) |
| ESR mm/h | 20.2 (±16.7) | 8.5 (±3.53) | p<0.0001** (unpaired T-test) |
| CRP mg/dL | 0.58 (±1.34) | 0.50 (±0.35) | n.s. 0.6554 (unpaired T-test) |
| DISH (percentage) | 28 patients (46%) | 10 patients (16%) | p=0.0007** (Fisher test) |
| Enthesitis (overall) | 52 patients (86%) | 8 patients (13%) | p<0.00001** (Fisher test) |
| Global inflammatory US score | 2.1 (±1.44) | 0.1 (±0.61) | p<0.0001** (unpaired Welch corrected T-test,) |
| Global structural damage US score | 3.5 (±1.41) | 0.6 (±4.24) | p<0.0001** (unpaired Welch corrected T-test) |
| Erosions | 2 patients (2 entheses) | 0 patients (0 entheses) | p=0.4958ns (Fisher test) |
| Power Doppler signals | 9 patients (11 entheses) | 1 patients (1 enthesis) | p=0.0166* (Fisher test) |
| Localization of enthesitis | |||
| Inflammatory changes | |||
| Acromial deltoid insertion | 2 | 0 | p=0.459 n.s. (Fisher test) |
| Supraspinatus enthesis | 10 | 2 | p<0.0001** (Fisher test) |
| Lateral epicondyle enthesis | 21 | 0 | p<0.0001** (Fisher test) |
| Medial epicondyle enthesis | 1 | 0 | p=1.0 n.s. (Fisher test) |
| Olecranic triceps enthesis | 0 | 0 | p=1.0 n.s. (Fisher test) |
| Trochanteric enthesis | 4 | 0 | p=0.118 n.s. (Fisher test) |
| Quadriceps insertion | 19 | 0 | p<0.0001** (Fisher test) |
| Patellar tendon (proximal) | 1 | 0 | p=1.0 n.s. (Fisher test) |
| Patellar tendon (distal) | 2 | 0 | p=495 n.s. (Fisher test) |
| Ileo-tibial tract insertion | 0 | 0 | p=1.0 n.s. (Fisher test) |
| Achilles tendon enthesis | 13 | 0 | p<0.0001** (Fisher test) |
| Plantar fascia enthesis | 50 | 2 | p<0.0001** (Fisher test) |
| Structural damages | |||
| Acromial deltoid insertion | 0 | 0 | p=1.0 n.s. (Fisher test) |
| Supraspinatus enthesis | 17 | 5 | p=0.0084** (Fisher test) |
| Lateral epicondyle enthesis | 25 | 2 | p<0.0001** (Fisher test) |
| Medial epicondyle enthesis | 3 | 0 | p=0.843 n.s. (Fisher test) |
| Olecranic triceps enthesis | 14 | 0 | p<0.0001** (Fisher test) |
| Trochanteric enthesis | 10 | 2 | p=0.0295* (Fisher test) |
| Quadriceps insertion | 35 | 7 | p<0.0001** (Fisher test) |
| Patellar tendon (proximal) | 2 | 0 | p=1.0 n.s. (Fisher test) |
| Patellar tendon (distal) | 7 | 0 | p=0.013* (Fisher test) |
| Ileo-tibial tract insertion | 0 | 0 | p=1.0 n.s. (Fisher test) |
| Achilles tendon enthesis | 52 | 17 | p<0.0001** (Fisher test) |
| Plantar fascia enthesis | 48 | 7 | p<0.0001** (Fisher test) |
Data are expressed as mean (±standard deviation, SD), if not otherwise specified. The level of statistical significance was set at a p-level of 0.05. ns: not significant, *p<0.05, **p<0.01. US: ultrasound, MetS: metabolic syndrome, LEI: Leeds Enthesitis Index, BMI: body mass index, DISH: diffuse idiopathic hyperostosis syndrome, ESR: erytrosedimentation rate, CRP: C-reactive protein.
The patients with MetS showed moderate overweight (mean BMI 29.8) and a concurrent diagnosis of type 2 diabetes was present in 24 (40%). Moreover, in these patients a low grade inflammation was demonstrated (mean CRP 0.58mg/dL, normal values<0.5, mean ESR 20.2, normal values<20) with ESR values significantly higher than those of controls (p<0.0001) (Table 1).
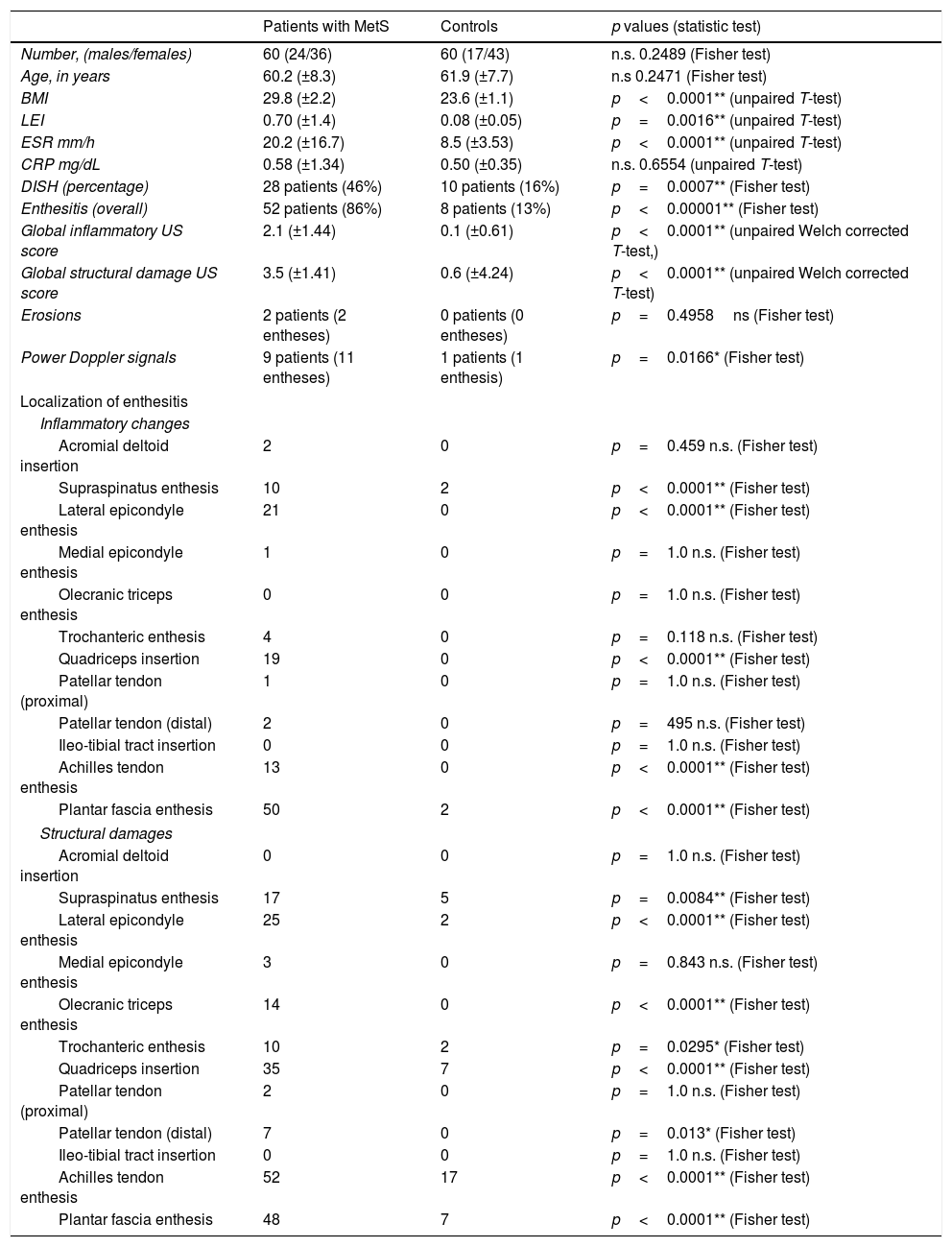
The PDUS appearance of dysmetabolic enthesopathies comprises all pathologic changes typical of enthesitis (thickening, hypoecogenicity, neovascularization), even if these abnormalities often spread from the enthesis toward the mid-portion of tendon, with a peculiar gross appearance of enthesophytes (Fig. 1).
Patellar tendon enthesopathic changes in patient with metabolic syndrome. Anterior longitudinal scan over the distal enthesis of patellar tendon, linear 6–18MHz probe. The distal enthesis of the patellar tendon (between arrowheads) is markedly thickened and hypoechoic, with gross enthesophytosis (arrow). Power Doppler analysis (within the color box) shows abundant neovascularisation in the distal third of the tendon. All the abnormalities spread beyond the real enthesis toward the body of the tendon.
We demonstrated a high prevalence of PDUS-defined enthesitis in 52 dysmetabolic patients (86% out of 60 patients) with 127 enthesitis (17.6% out of 720 entheses), and higher mean US inflammatory score than controls (p<0.0001) (Table 1). Moreover, in 57 patients (95% out of 60 patients) and 217 entheses (30% out of 720 entheses) structural damages have been found, with significant higher frequency than in controls (p<0.0001) (Table 1). The differences in location and number of affected entheses between the two groups are reported in Table 1.
Power Doppler signals, indicative of “active” enthesitis were reported in 9 patients (15% out of 60 patients, 1.52% out of 720 entheses). Power Doppler signals were significantly associated to both clinical symptoms expressed as LEI (r=0.3137, p=0.0138) and male gender (r=0.3643, p=0.0036)(Table 2). Power Doppler entheseal signals were significantly more frequent in MetS patients than in controls (p=0.0166) Entheseal erosions resulted rare in MetS group (2 patients, 0.3% of entheses) but absent in controls (Table 1).
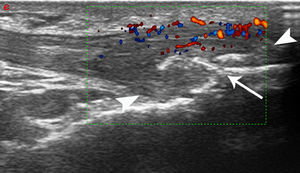
Clinical-anthropometric parameters of patients with metabolic syndrome and correlations with ultrasound findings.
| US findings | LEI | BMI | Type II diabetes | Gender males/females | Age in years | DISH | ESR | CRP | |
|---|---|---|---|---|---|---|---|---|---|
| MetS patients parameters | 0.7 (±1.4) | 29.8 (±2.2) | 24 (40%) | 24/36 | 60.2 (±8.3) | 28 (46%) | 20.2 (±16.7) | 0.58 (±1.34) | |
| Global inflammatoryUS score | 2.1 (±1.44) | r=0.2781p=0.03 * | r=0.2902p=0.0233* | p=0.0618ns | p=0.5307ns | p=0.8704ns | r=0.2762p=0.0312* | p=0.5919ns | p=0.2903ns |
| Global structural damageUS score | 3.5 (±1.41) | r=0.3278p=0.0099 ** | r=0.3428p<0.0068 ** | r=o.2638p=0.0399* | p=0.6738ns | p=0.8921ns | r=0.3414p=0.0071** | 0.5561ns | 0.0961ns |
| Power Doppler signals | 9 patients (11 entheses) | r=0.3137p=0.0138* | p=0.6853 | p=0.8036ns | r=0.3643p=0.0036 ** | p=0.3415ns | p=0.4425ns | 0.1642ns | 0.4051ns |
| Erosions | 2 patients (2 entheses) | p=0.1268 | p=0.6775 | p=0.7939ns | p=0.0764ns | p=0.2342ns | 0.9078ns | 0.7460ns | 0.2274ns |
Data are expressed as mean (±standard deviation, SD), if not otherwise specified. The non-parametric Spearman rank test was applied to correlate variables. The level of statistical significance was set at a p-level of 0.05. ns: not significant, *p<0.05, **p<0.01, US: ultrasound, MetS: metabolic syndrome, LEI: Leeds Enthesitis Index, BMI: body mass index, DISH: diffuse idiopathic hyperostosis syndrome, ESR: erytrosedimentation rate, CRP: C-reactive protein.
In MetS group a significant positive correlation has been found between US global inflammatory and BMI (r=0.2902, p=0.0233), and LEI (r=0.2781, p=0.03), while US structural damage scores showed a significant positive correlation with BMI (r=0.3428, p=0.0068), LEI (r=0.3278, p=0.0099) and presence of type 2 diabetes (r=0.2638, p=0.0399) (Table 2).
In control group a diagnosis of PDUS-defined enthesitis could be made in 8 subjects (13%), with mean US global inflammatory score 0.13±0.6 and mean US global structural damage score of 0.60±4.2 (Table 1).
In 28 MetS patients (46%) a diagnosis of DISH could be made on the basis of spinal radiography. In a model of multivariate regression analysis the best predictors of the presence of DISH (r squared 0.4506) were higher levels of CRP (t=2.128, p=0.038) and older age (t=5.129, p<0.0001). Spearman rank test demonstrated significant direct correlation between the presence of DISH and older age (r=0.5824, p<0.0001), inflammatory reactants (r=0.2603, p=0.0428 for CRP, r=0.3423 p=0.0069 for ESR). Also the US enthesitis scores were significantly associated to the presence of DISH (r=0.2762, p=0.0312 for inflammatory damage, r=0.3414, p=0.0071 for structural damage) (Table 2). Also in 10 control subjects (16%) a diagnosis of DISH could be made on the basis of spinal radiography. Spearman rank test demonstrated significant direct correlation between the presence of DISH and older age (r=0.6324, p<0.0001), male gender (r=0.3173, p=0.0127), but not with inflammatory reactants, nor US scores.
The presence of MetS had a strong association (Odd Ratio [OR] 4.375 [95% confidence interval 1.874–10.212], p=0.0007) with concurrent DISH.
DiscussionThe majority of works about US-defined enthesitis are focused in SpA-related enthesitis, and scanty data are available about entheseal involvement in metabolic diseases as obesity, type 2 diabetes or metabolic syndrome, characteristically considered as not-inflammatory disorders.3,8,10,11 In the other hand all these dysmetabolic conditions showed strong associations with the development of DISH, a senile condition characterized by ossification and calcification of peripheral and spinal entheses.5,15 Previous study confirmed the role of overweight in plantar enthesopathy16 and the association of type 2 diabetes in midportion tendinopathy and enthesopathy of Achilles tendon.3,11,17,18 This is the first study in which diffuse peripheral entheseal abnormalities are demonstrated in MetS with multi-site PDUS examination.
Both US inflammatory and damage scores for enthesitis showed a direct correlation with overweight and type 2 diabetes. In diabetes has been hypothesized that an increased production of advanced glycation end products could deteriorate mechanical structure and function of tendon, via cross-linking within collagen fibers. On the other hand an excessive adipose tissue can promote tendon damages with both mechanical (overweight) and immunological (leptin, adiponectin, serum amyloid) pathways.19,20 Adipose tissue is now recognized as an endocrine organ able to secrete adipose-derived factors named adipokines.7 Adipokines have been linked to the pathogenesis of MetS and its comorbidities through their effects on vascular function and inflammation.19 The present study confirm the strict association of MetS with both mild inflammatory state, and significant higher frequency of entheseal abnormalities.
Moreover, in MetS patients, entheseal abnormalities resulted not limited to tendons with higher biomechanical charge. In fact also upper limbs entheseal sites (especially supraspinatus and lateral epicondyle entheses) were involved by relevant enthesopathic changes.
On the other hand, in the control group most of the enthesopathies was localized in the lower limbs. These observations confirm that enthesitis of MetS can be considered a systemic process, and not only promoted by biomechanical factors.
Tendinopathies and enthesopathies of dysmetabolic diseases are classically defined not inflammatory conditions11 in contrast with the primary immunological SpA-related enthesitis, in which entheseal inflammation seems to be the first event of the pathological changes. As hystologic early changes of enthesitis are hardly studied, the definition of inflammatory signs of enthesitis are surrogated by imaging. In particular bone marrow subentheseal edema in MRI, and entheseal vascularization on PDUS seem to be some of the most suggestive signs of primary immunonogical enthesitis, even if not specific.11,21–23 In our study entheseal vascularization on PDUS has been reported in a small quote of patients (15%), but always accompanying structural abnormalities, suggesting that neovascularisation of enthesitis could have both inflammatory and also reparative significance, as suggested in previous work.24 The frequency of entheseal vascularization in our dysmetabolic patients is comparable with those reported in SpA-related enthesitis.22,23,25–27 On the other hand dysmetabolic patients had a mild inflammatory state that could contribute to entheseal inflammation.7,19
Our data confirmed that power Doppler positivity is significantly associated to entheseal pain expressed as LEI, even if this clinical index is limited to few entheses, as confirmed in previous works.22,23 This aspect suggests that neuro-vascular entheseal ingrowth could contribute to explain pain associated to neovascularisation in entheseal affected sites.24
In our work we demonstrated a high frequency of PDUS-defined enthesitis in dysmetabolic patients, using the most recent US definition of enthesitis. As these frequencies are comparable or also higher than those reported for SpA-related enthesitis,22,23,25–27 these data suggest a low specificity of this definition, as reported in recent observations.28 A more specific US definition of enthesitis should comprises a combination of gray-scale and PD findings. In particular, erosions and PD signal should be differently weighted, as yet proposed in a recent enthesitis score.29 In fact, also in the present study, in dysmetabolic patients and normal subjects the frequencies of PD signals and erosions were relatively low. Moreover, the US appearance of neovascularization and enthesopatic changes should be also considered beyond the first 2mm from the enthesis. In dysmetabolic enthesopathies we can observe pathologic changes (thickening, hypoecogenicity, neovascularization) spreading from the enthesis toward the body of tendon and the peculiar thickening involving the mid-portion of tendon can reinforce the dysmetabolic pathogenesis.18
Finally, this study demonstrated that almost half of patients with MetS could have a concurrent diagnosis of DISH on the basis of spinal radiography, and this data is comparable with those obtained in previous works (DISH diagnosis in 47–64% of MetS).5 The association between age, male gender and DISH has been confirmed also in our control group.
In a previous study in which DISH was diagnosed in symptomatic patients with low back pain, the prevalence of the disease was about 30% in the age range between 65 and 70 years, using the same criteria.30 The prevalence of DISH in our patients group is resulted higher (46%) than expected according to age and, at the same time, the prevalence of DISH in control group is resulted lower than expected,30 and this can be related to our strict criteria of normality. The higher prevalence of DISH in our case group could be directly related to the dysmetabolic condition, and this strong association between DISH and MetS (OR 4.375) is comparable with those reported in previous works (OR 3.88–3.61).5
Moreover patients with DISH are significantly older, with higher levels of inflammation, and they have higher scores of PDUS-defined enthesitis. Even if these data should be confirmed by longitudinal studies, our results suggest that enthesopathy of MetS and DISH could be strictly related, and considered as progressive aspects of the same pathology. Diffuse enthesitis and low grade inflammatory state should be regarded as potential factors associated to progression from MetS toward a conclamed DISH, and further longitudinal studies are required to clarify the present understanding of the pathogenesis of DISH.
Conflict of interestAuthors declare no potential conflict of interest.