Osteoporosis causes significant morbidity and mortality by the development of fragility fractures, including vertebral fractures. Patients with gout may show an increased risk of osteoporotic fractures, as accelerated bone resorption is likely linked to urate crystal-led inflammatory state. This study aims to evaluate the risk of osteoporotic dorsal vertebral fractures associated with gout.
MethodsCross-sectional study carried out in patients admitted for cardiovascular events. Patients with available lateral view of chest radiography (on admission or in the previous six months) were selected. Two observers blinded to clinical data reviewed the radiographies simultaneously. Vertebral fracture was defined as a vertebral height loss ≥20%, and presence, number, and severity (by Genant semi-quantitative scale) were registered. To analyse the relationship between gout and the presence of vertebral fractures, the odds ratio (OR) with 95% confidence interval (95%CI) was calculated by multiple logistic regression.
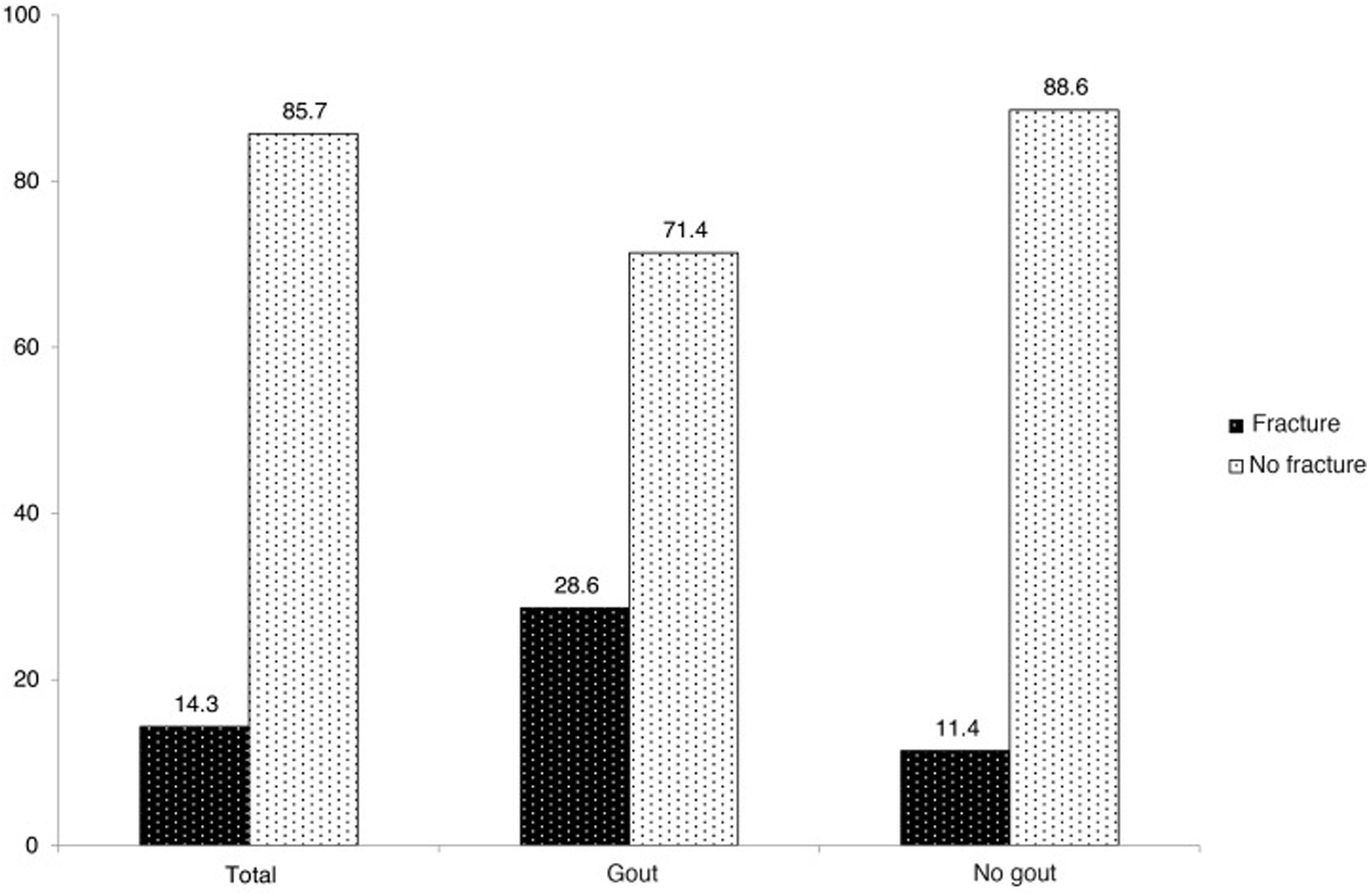
Results126 patients were analysed, 21 of them (16.67%) suffered from gout. Eighteen cases with fractures were detected, with a prevalence of 14.3%. A significant association was found between gout and vertebral fracture (28.6% gout, 11.4% controls; OR 3.10, 95%CI 1.01–9.52). There were no differences in the number of fractures, while the severity was found to be higher in the controls. The association between gout and vertebral fracture persisted after multivariate adjustment (OR 5.21, 95% CI 1.32−20.61).
ConclusionAn independent association between gout and radiological thoracic vertebral fractures was revealed in patients with a cardiovascular event.
La osteoporosis causa gran morbilidad y mortalidad por el desarrollo de fracturas por fragilidad, entre ellas las vertebrales. Los pacientes con gota podrían mostrar un incremento de riesgo de fracturas osteoporóticas debido a una mayor resorción ósea por un estado inflamatorio producido por los cristales de urato. El objetivo de este estudio fue evaluar el riesgo de fracturas vertebrales dorsales osteoporóticas asociado a padecer gota.
MétodosEstudio transversal realizado con pacientes ingresados por evento cardiovascular. Se seleccionaron pacientes con radiografía torácica lateral reciente al ingreso o en los seis meses previos, que fueron revisadas de forma simultánea por dos observadores desconocedores de los datos clínicos. Se definió fractura vertebral como reducción de la altura vertebral ≥20%, registrando su presencia, número y grado mediante la escala semicuantitativa de Genant. Para analizar la relación entre gota y fractura vertebral, se calculó la odds ratio (OR) con intervalo de confianza al 95% (IC95%) mediante regresión logística múltiple.
ResultadosSeleccionamos 126 pacientes, de los que 21 (16,67%) padecían gota. Se detectaron 18 casos con fracturas, siendo la prevalencia 14,3%. Se encontró una asociación estadísticamente significativa entre gota y fractura vertebral (28,6% gota, 11,4% no gota; OR 3,10, IC95% 1,01–9,52). No hubo mayor número de fracturas por grupos, y la severidad fue superior en los controles. La asociación entre gota y fractura vertebral persistió tras ajuste multivariante (OR 5,21, IC95% 1,32−20,61).
ConclusiónSe ha identificado una asociación independiente entre gota y fracturas vertebrales dorsales radiográficas en pacientes con evento cardiovascular.
Gout is a disease caused by the deposit of monosodium urate crystals in articular, periarticular and subcutanous areas. Deposits are persistent but potentially reversible with the normalisation of uricaemia. It is the most common type of arthritis, clinically manifested as recurrent episodes of acute arthritis. Hyperuicaemia is a necessary condition but insufficient, for the appearance of gout, with the threshold being a serum urate concentration above 7 mg/dL1,2.
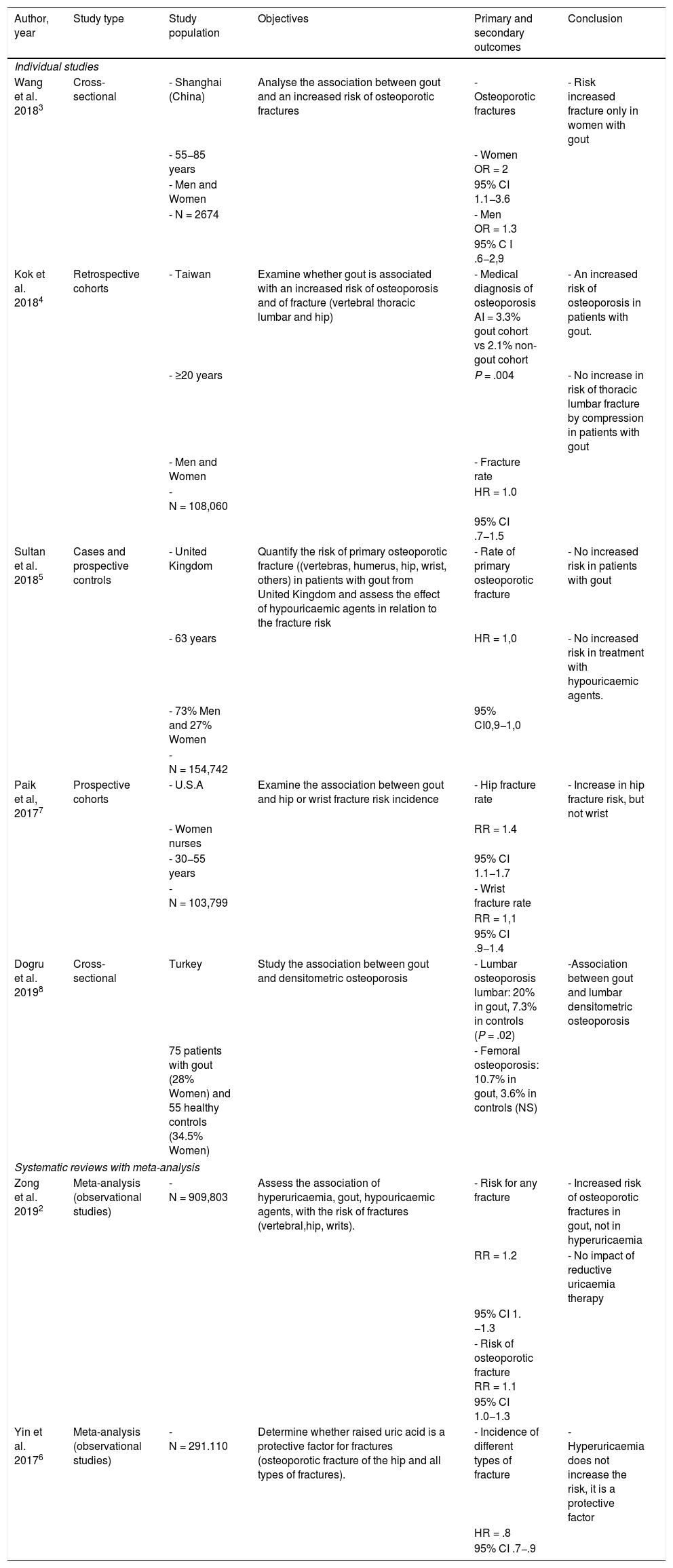
Up until now, the relationship between gout and metabolic bone disease is unclear. Several studies have shown that uric acid may affect bone homeostasis but results have been contradictory on whether it has a protective or damaging effect on bone remodelling2–8. Monosodium urate crystals are recognised by the innate immune system and produce inflammation through the activation of inflammasome NLRP3, inducing the synthesis and secretion of proinflammatory cytokines (IL-1, IL-6, TNF alpha). These cytokines have been linked to the stimulation of bone resorption and inhibition of bone formation8. Hyperuricaemia also appears to interrupt the activity of 1-alph-hydroxilase, leading to a 1.25(OH)2-vitamin D drop and an increase in the parathormone, which implies higher bone resorption3,4,7. The association between gout and osteoporotic fracture is a controversial issue in clinical studies and the available literature (Table 1) shows mixed results. Equally, it should be specified whether there may be differences between medical gout (and its characteristics and evolution) or asymptomatic hyperuricaemia, also analyzing the effect of treatment with hypouricaemic agents. Studies in more homogenous population groups are also required. Geographic location may compromise external validity of outcomes, either due to quality of life or diet. Other accompanying factors may also notably affect the appearance of fractures, such as cardiovascular disease, tobacco, alcohol, physical activity, or kidney function.
Previous published studies which assess the association of gout, osteoporosis and fractures.
| Author, year | Study type | Study population | Objectives | Primary and secondary outcomes | Conclusion |
|---|---|---|---|---|---|
| Individual studies | |||||
| Wang et al. 20183 | Cross-sectional | - Shanghai (China) | Analyse the association between gout and an increased risk of osteoporotic fractures | - Osteoporotic fractures | - Risk increased fracture only in women with gout |
| - 55−85 years | - Women OR = 2 | ||||
| - Men and Women | 95% CI 1.1−3.6 | ||||
| - N = 2674 | - Men OR = 1.3 | ||||
| 95% C I .6−2,9 | |||||
| Kok et al. 20184 | Retrospective cohorts | - Taiwan | Examine whether gout is associated with an increased risk of osteoporosis and of fracture (vertebral thoracic lumbar and hip) | - Medical diagnosis of osteoporosis AI = 3.3% gout cohort vs 2.1% non-gout cohort | - An increased risk of osteoporosis in patients with gout. |
| - ≥20 years | P = .004 | - No increase in risk of thoracic lumbar fracture by compression in patients with gout | |||
| - Men and Women | - Fracture rate | ||||
| - N = 108,060 | HR = 1.0 | ||||
| 95% CI .7−1.5 | |||||
| Sultan et al. 20185 | Cases and prospective controls | - United Kingdom | Quantify the risk of primary osteoporotic fracture ((vertebras, humerus, hip, wrist, others) in patients with gout from United Kingdom and assess the effect of hypouricaemic agents in relation to the fracture risk | - Rate of primary osteoporotic fracture | - No increased risk in patients with gout |
| - 63 years | HR = 1,0 | - No increased risk in treatment with hypouricaemic agents. | |||
| - 73% Men and 27% Women | 95% CI0,9−1,0 | ||||
| - N = 154,742 | |||||
| Paik et al, 20177 | Prospective cohorts | - U.S.A | Examine the association between gout and hip or wrist fracture risk incidence | - Hip fracture rate | - Increase in hip fracture risk, but not wrist |
| - Women nurses | RR = 1.4 | ||||
| - 30−55 years | 95% CI 1.1−1.7 | ||||
| - N = 103,799 | - Wrist fracture rate | ||||
| RR = 1,1 | |||||
| 95% CI .9−1.4 | |||||
| Dogru et al. 20198 | Cross-sectional | Turkey | Study the association between gout and densitometric osteoporosis | - Lumbar osteoporosis lumbar: 20% in gout, 7.3% in controls (P = .02) | -Association between gout and lumbar densitometric osteoporosis |
| 75 patients with gout (28% Women) and 55 healthy controls (34.5% Women) | - Femoral osteoporosis: 10.7% in gout, 3.6% in controls (NS) | ||||
| Systematic reviews with meta-analysis | |||||
| Zong et al. 20192 | Meta-analysis (observational studies) | -N = 909,803 | Assess the association of hyperuricaemia, gout, hypouricaemic agents, with the risk of fractures (vertebral,hip, writs). | - Risk for any fracture | - Increased risk of osteoporotic fractures in gout, not in hyperuricaemia |
| RR = 1.2 | - No impact of reductive uricaemia therapy | ||||
| 95% CI 1. −1.3 | |||||
| - Risk of osteoporotic fracture RR = 1.1 | |||||
| 95% CI 1.0−1.3 | |||||
| Yin et al. 20176 | Meta-analysis (observational studies) | -N = 291.110 | Determine whether raised uric acid is a protective factor for fractures (osteoporotic fracture of the hip and all types of fractures). | - Incidence of different types of fracture | -Hyperuricaemia does not increase the risk, it is a protective factor |
| HR = .8 | |||||
| 95% CI .7−.9 | |||||
AI: Accumulated Incidence; 95% CI: 95% Confidence Interval; HR: Hazard Ratio; NS: Not significant; OR: Odds Ratio; RR: Relative Risk.
Osteoporotic vertebral fractures are linked to an increase in morbimortality and to significant social and financial burden, and their prevention is therefore essential in the public health9,10 context. On many occasions, these fractures occur without pain and go unnoticed, and their study through studies with large population groups is therefore challenging. In fact, with regard to gout, the study by Kok et al.4 conducted in Taiwan, is one of the few that analyse vertebral fractures. The authors did not detect an increased risk in patients with gout, but again, these fractures were taken from medical records, and the possibility of major under-recording existed. It would therefore be interesting to establish the prevalence and risk of osteoporotic vertebral fractures in patients with gout, using subclinical fracture detection strategies.
For this study, we began with the hypothesis that patients with gout will present with an increased risk of an osteoporotic type vertebral fracture, with an independent association. The main objective of our study was to assess the risk of osteoporotic dorsal vertebral fractures, determined through lateral thoracic radiography, associated with suffering from gout. Our secondary objectives were to discover the impact of other patient factors such as gender or age group in the association with gout, together with the possible impact of disease control level and specific treatment.
Material and methodsType of study and populationA cross-sectional or prevalence observational, analytical study conducted in the General University Hospital of Alicante. This study was approved by the local ethics committee (ref. 180179).
The study population were patients included in a previous study11, who were hospitalised for cardiovascular events (acute coronary syndrome or coronary arterial disease, de novo heart failure or decompensated heart failure, stroke or transient ischaemic attack and acute peripheral arterial disease or chronic revascularised arterial disease) in the cardiology, neurology and vascular surgery units of the hospital, selected by systematic non-consecutive sampling and interviewed to estimate the prevalence of gout in this population. The total number of patients recruited in the study was 266, and the recruitment period was from January to October 2018. Patients were grouped according to the diagnosis of gout, which was established using a review of medical records and a structured interview, in 40 patients with gout and 226 patients without gout.
For secondary analysis, participants with lateral thoracic radiography were selected. These were available due to hospitalization for cardiovascular events or, if they were not, the most recent radiographs from the previous six months were selected. No additional exclusions criteria were applied.
VariablesIn this study the presence of dorsal vertebral fractures was assessed using lateral thoracic radiography. It was assumed that the fractures detected at thoracic level were mainly of osteoporotic type, being the type of fracture most intricately connected to osteoporosis. Several studies demonstrated that over 90% of these fractures are related to a low mineral bone density, and only a minor percentage was attributed to malignant traumatic causes12–14.
The main study variable was prevalence of vertebral fracture, coded as a dichotomous variable (fracture yes/no). A vertebral fracture was considered to be one where there was a loss of height equal to or higher than 20% with regard to total height14. The following was also determined: 1) the number of existing fractures and 2) using the Genant scale, the method of assessment with which the vertebral shape was observed (wedge, concave or crush fracture), and the existing reduction in the internal vertebral height, posterior and/or mean. The severity of the fracture was established in three groups: mild or grade 1 (loss of height between 20%–25%), moderate or grade 2 (loss of height between 25%–40%) and severe or grade 3 (loss of height above 40%)15. The Genant scale showed good intra and interobserver consistency for the evaluation of vertebral fractures.
With regard to independent variables the presence of gout was recorded (defined by clinical criteria and/or through a microscope, according to ACR/EULAR16,17), its characteristics and associated treatments. Age in years was also recorded, and subsequently classified into terciles (T1, T2 and T3), BMI (in kg/m2), sex, cardiovascular risk factors (high blood pressure, diabetes mellitus, dyslipidaemia, tobacco, alcohol), previously established cardiovascular disease (acute coronary syndrome or coronary arterial disease, de novo heart failure or decompensated heart failure, stroke or transient ischaemic attack and acute peripheral or chronic revascularised arterial disease), the presence of chronic kidney disease (glomerular filtrate < 60 mL/min/1.73 m2) and use of hyperuricaemic diuretics (thiazides or loop).
ProceduresLateral chest radiographies performed on the patients included in the study were reviewed retrospectively to assess the presence, number, and severity of vertebral fractures. The radiography closest to the date of inclusion in the study was selected. When the said technique during admission was unavailable, electronic register was reviewed and the latest one available from the previous six months was taken. The review was simultaneously carried out by two observers, who were blinded to the clinical data of the patients. The discrepancies were discussed with a third researcher (MA) and resolved jointly, always blinded to patients’ clinical data. The observers had previously had specific online training with the International Osteoporosis Foundation (IOF) called “Vertebral fracture teaching program” to help with imaging comprehension and diagnosis for this type of fractures in clinical practice. This facilitated thoroughness and minimized errors in assessment of patient radiographies17.
Analysis planFrequencies and percentages were used to express the main qualitative variable (prevalence of vertebral osteoporotic thoracic fracture). Regarding quantitative variables, these were expressed as central tendency measures, such as mean and median and dispersion measures such as standard deviation and 25−75 percentile.
For risk estimation between gout and vertebral fracture, the odds ratio (OR) was calculated with a 95% confidence interval (95% CI). The χ2 test was used to compare the different grades in the Genant scale of the fractures present between study groups. Also, the Mann-Whitney U test was used to compare the number of existing fractures. The χ2 and exact Fisher test were also used to analyse the categorical independent variables and for quantitative variables the Mann-Whitney U test was used (age, body mass index). Following this, a multivariate logistic regression model was constructed, analysing independent variables (gout and chronic kidney failure), with significant association with the dependent variable of those considered to be clinically relevant (age, female sex), to analyse the effect of confusion factors.
Statistical analysis was performed using the SPSS v25 (IBM, Armonk, NY) software programme.
For this study a statistical significance level under 0.05 was used.
ResultsOf the 266 patients in the study, lateral thoracic radiography was available in 126 patients (47.4%), who were selected for this analysis. There were no differences in availability of radiographies by study groups (52.5% in gout versus 46.5% in non-gout, P = .481). The patients included or excluded according to the availability of the lateral chest radiographies showed comparable clinical characteristics (Appendix B, supplementary material, Table 1S).
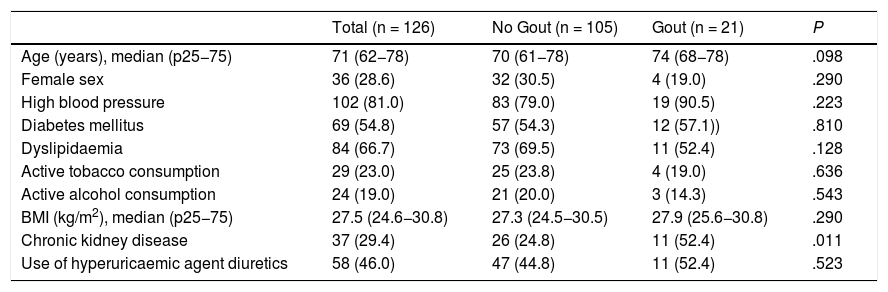
Table 2 shows the total population and study groups. The groups were globally comparable, except regarding the presence of chronic kidney disease, which was higher in those with gout. Different characteristics were collected relating to gout, which are shown in Table 3.
Clinical characteristics of the study population and comparison by groups of interest. Data shown as n (%), except when specified to the contrary.
| Total (n = 126) | No Gout (n = 105) | Gout (n = 21) | P | |
|---|---|---|---|---|
| Age (years), median (p25−75) | 71 (62−78) | 70 (61−78) | 74 (68−78) | .098 |
| Female sex | 36 (28.6) | 32 (30.5) | 4 (19.0) | .290 |
| High blood pressure | 102 (81.0) | 83 (79.0) | 19 (90.5) | .223 |
| Diabetes mellitus | 69 (54.8) | 57 (54.3) | 12 (57.1)) | .810 |
| Dyslipidaemia | 84 (66.7) | 73 (69.5) | 11 (52.4) | .128 |
| Active tobacco consumption | 29 (23.0) | 25 (23.8) | 4 (19.0) | .636 |
| Active alcohol consumption | 24 (19.0) | 21 (20.0) | 3 (14.3) | .543 |
| BMI (kg/m2), median (p25−75) | 27.5 (24.6−30.8) | 27.3 (24.5−30.5) | 27.9 (25.6−30.8) | .290 |
| Chronic kidney disease | 37 (29.4) | 26 (24.8) | 11 (52.4) | .011 |
| Use of hyperuricaemic agent diuretics | 58 (46.0) | 47 (44.8) | 11 (52.4) | .523 |
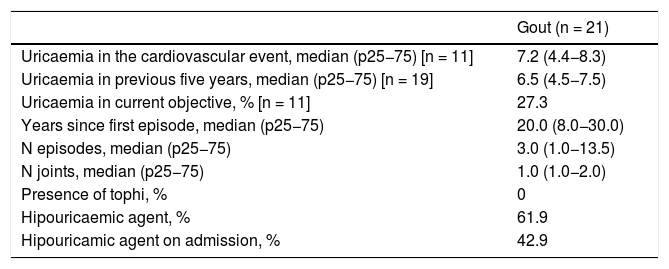
Clinical characteristics and treatment of group with gout.
| Gout (n = 21) | |
|---|---|
| Uricaemia in the cardiovascular event, median (p25−75) [n = 11] | 7.2 (4.4−8.3) |
| Uricaemia in previous five years, median (p25−75) [n = 19] | 6.5 (4.5−7.5) |
| Uricaemia in current objective, % [n = 11] | 27.3 |
| Years since first episode, median (p25−75) | 20.0 (8.0−30.0) |
| N episodes, median (p25−75) | 3.0 (1.0−13.5) |
| N joints, median (p25−75) | 1.0 (1.0−2.0) |
| Presence of tophi, % | 0 |
| Hipouricaemic agent, % | 61.9 |
| Hipouricamic agent on admission, % | 42.9 |
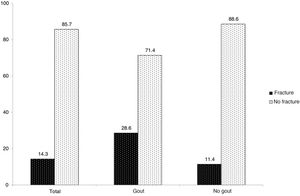
Eighteen cases with fractures were detected, with global fracture prevalence in the study population standing at 14.3%. Of these, six patients belonged to the gout group (28.6% of the total of this group) whilst 12 patients belonged to the non-gout group (11.4% of the total of this group) (Fig. 1). Regarding the comparison by groups, a statistically significant association was found between gout and vertebral fracture (OR 3.1, 95% CI 1.01–9.52).
In a prevalence analysis according to age tercile, the results found were as follows: in tercile 1 (≤64 years), there was a fracture prevalence of 50.0% in the gout group compared with a 7.5% fracture prevalence in the non-gout group (P = .184). In tercile 2 (64.1–74.0 years), fracture prevalence was 30.0% in the gout group compared with 6.9% in the non-gout group (P = .096). And in tercile 3 (≥74.1 years), there was a fracture prevalence of 22.2% in the gout group and of 19.4% in the non-gout group (P = 1.000).
In the sub-analysis by sex, fracture prevalence in women with gout was 50.0% compared with 28.1% in women who did not present with gout (P = .570). Regarding fracture prevalence of men with gout, this was 23.5%, compared with 4.1% in the men who did not have gout (P = .022).
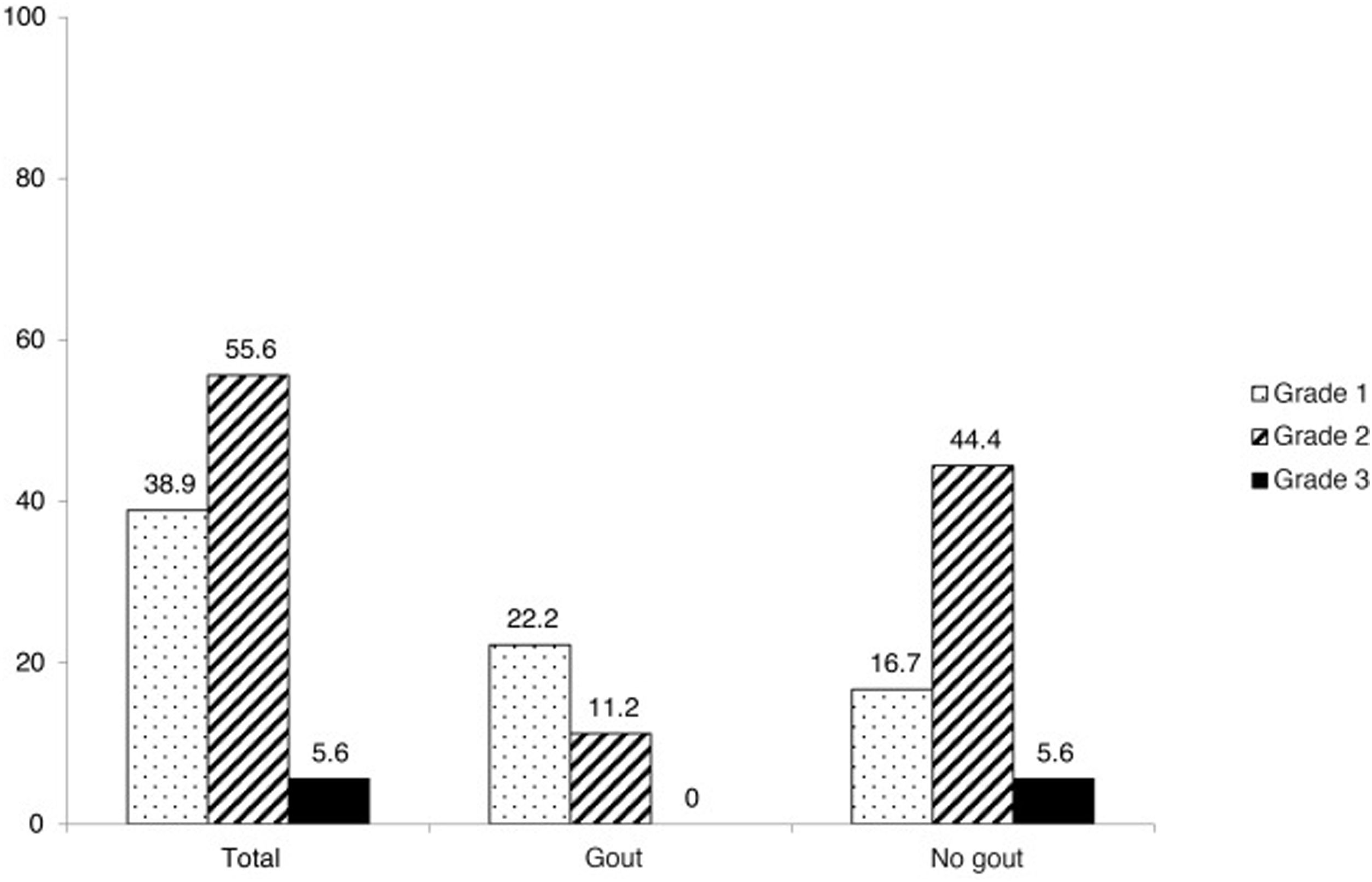
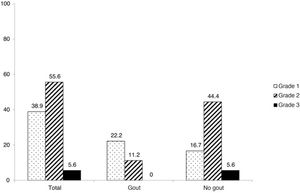
With regards to the number of fractures per patient, the median of fractures was 0 (p25–75 0–0) for the group without gout and 0 (0–1) for the group with gout, with no statistically significant differences being obtained (P = .051). The maximum number of fractures per patient found for each group was four for the group without gout and two for the group with gout. Regarding severity of fractures according to the Genant scale, several patients presented with grade 1 fractures (38.9%), 10 patients with grade 2 (55.6%) and one patient with grade 3 (5.6%) (Fig.2). These scores on the Genant scale showed differences by groups (P = .028): in gout, 22.2% grade 1, 11.1% grade 2 and none grade 3, whils5 in the group without gout, 16.7% showed a grade 1, 44.4% grade 2, and 5.6% grade 3).
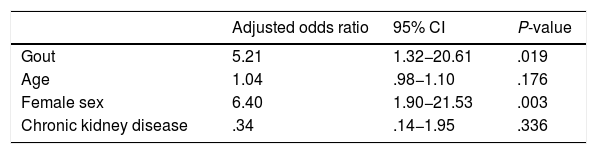
Apart from gout, multivariate analysis included age, female sex and chronic kidney disease as covariables. The results are contained in Table 4: suffering from gout had an independent statistically significant association with the presence of radiographic dorsal vertebral fracture.
Multiple logistic regression model for assessing association with the outcome variable (radiographic presence thoracic vertebral fracture).
| Adjusted odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Gout | 5.21 | 1.32−20.61 | .019 |
| Age | 1.04 | .98−1.10 | .176 |
| Female sex | 6.40 | 1.90−21.53 | .003 |
| Chronic kidney disease | .34 | .14−1.95 | .336 |
In this cross-sectional study conducted in patients who were admitted to hospital due to a cardiovascular event, the presence of a radiographic prevalence of dorsal vertebral fracture which could be considered high (14.3%) as determined. Furthermore, a statistically significant association between gout and presenting with a radiographic dorsal vertebral fracture was identified. This risk persisted despite adjustment of confusion factors, which suggest it is a direct and independent factor. In the group of patients with gout, the number of fractures was only higher numerically, but with a lower degree of severity according to the Genant scale. Since this was a selected sample, these results must be confirmed in larger populations with gout and with no cardiovascular-associated event.
In both sexes there was a higher percentage of patients who had suffered from fracture in the group with gout, but this difference was only significant in the men. The fracture rate in men without gout with a cardiovascular event was the lowest of the series (4.1%). This was probably due to the low frequency of other osteopenia-induced factors in these patients, since cardiovascular disease in itself is not considered as such18. In fact, there seems to be a strong inverse link, relating osteoporosis with the appearance of atherosclerosis and the development of cardiovascular events18–20.
Regarding the three age groups, the percentage of patients with fracture was higher in those who suffered from gout. The comparison did not achieve significance probably due to the small size of each group separately. The detection of fractures in the youngest population group showed the rate of inflammation in the development of osteoporosis and fractures, as occurred in other diseases21,22. A high inflammatory load in one patient could be considered as indication for screening for osteoporosis and fractures, despite the absence of other risk factors. Up until now, the only inflammatory disease considered as such is rheumatoid arthirits23,24, and this could be another one of these.
The small sample size in the group of patients with gout prevented sub-analysis on the impact of disease control (attacks of gout or hypouricaemic agent treatment). None of the patients presented with tophi, indicative of poorly treated gout; together with the inflammatory load of tophaceous gout25, the fractures in this group could also be associated with local activation of osteoclasts induced by monosodium urate crystals26. A recent study concluded that treatment with hypouricaemic agents prescribed early in the course of the disease did not appear to affect long-term fracture risk5. Along these same lines, hyperuricaemia was not associated with fracture risk whilst suffering from gout was2,5. Also, the presence of urate crystals in asymptomatic hyperuricaemia, estimated to be approximately 20% of patents, was not assessed with this perspective to date. The said crystals, though inflammatory mediators, could accelerate osseous resorption5, with their situation possibly being comparable to clinical gout.
The strengths of this study are that the researchers had previous training, endorsed by the IOF, to analyse and assess radiographies systematically. When the radiography was assessed, they were unaware of any patient characteristics, and possible classification bias was therefore avoided. The use of the Genant scale as a semi-quantitative method was also of note, as this is the most useful and commonly used scale for assessing these osteoporotic fractures, with few intra and interobserver differences.
The patients with gout most commonly presented with metabolic syndrome factors (high blood pressure, dyslipidaemia, obesity), cardiovascular disease and kidney disease27,28. However, in this study only a higher prevalence of chronic kidney disease was detected. This was probably due to the study population (hospitalised, mostly male and with a cardiovascular event), which was based on a high prevalence of standard risk factors29. Extrapolation of results may therefore be limited. Furthermore, the relationship between hyperuricaemia-gout and kidney disease is very close, with pathogenic and prognostic implications between both entities30,31. In the population hospitalized due to cardiovascular events, when no uricaemia was present, kidney disease was an independent prediction of the presence of gout11.
Regarding limitations, the main one was the significant reduction in sample size due to the low availability of lateral thoracic radiographies, despite patients being hospitalised due to a cardiovascular event. However, the said losses were similar in both groups which also had comparable characteristics. Assessment of fractures was carried out on thoracic radiographies, not aimed at the specific examination of the spine. This could have impacted when interpreting them, although previous studies endorsed the use of lateral thoracic radiographies for the identification of fractures, which were mainly asymptomatic and therefore went unnoticed12,14. Furthermore, many patients received diuretic treatment, which may also have had an impact on the development of osteoporosis and fractures. Thiazide diuretics may attenuate bone loss stimulating re-absorption of calcium in the distal tubule, whilst loop diuretics have an opposite effect32. It should be borne in mind that the number of patients who presented with gout was small in this subpopulation.
The importance of this study is that if posterior studies confirm the association between gout and dorsal vertebral fracture, an active fracture search or a screening using densitometry for those patients with gout could be performed, identifying them and treating them in time to prevent major morbimortality resulting from osteoporotic fracture. In fact, osteoporosis is still not one of the comorbidities to be assessed in patients with gout according to EULAR33 experts. In future research studies the relationship between uricaemic control and the presentation of fractures could be analysed depending on the level of uric acid in each patient. Regarding new studies it would be pertinent to increase the number of patients in the gout group to be able to make more consistent comparisons, including a larger number of women and analyse fractures at other levels.
ConclusionFracture prevalence is increasing in patients who are hospitalized due to a cardiovascular event and who present with gout, with this association being independent from other variables. This increase in fracture risk in this group was appreciated for both sexes, although it was only significant in men (where the inflammatory load could be of greater impact). Furthermore, an increase in fractures in younger age groups was detected, which reinforces the role of inflammation as an osteopenia-inducing factor. The influence of control of uricaemic levels in the appearance of osteoporotic fractures remains to be clarified. Although this must be confirmed in future research it could be considered as the screening for osteoporosis and fractures in patients with gout and cardiovascular disease.
FinancingThis study received financing from the Alicante Institute of Health and Biomedical Research (5th call for aid for the support and promotion of research, file number 180179).
Conflict of interestsMA declares he received fees as a speaker and research funding from Grünenthal and Menarini. The other authors have no conflict of interests to declare with regard to this research study.
To Antonio Palazón Bru for his statistical assistance.
Please cite this article as: Ferrández-Jiménez M, Calabuig I, Peral-Garrido M-L, Gómez-Garberí M, Andrés M. Riesgo de fracturas vertebrales dorsales osteoporóticas en pacientes con gota. Reumatol Clin. 2022;18:279–285.