Off-label (OL) drug use is the prescription of a drug for indications other than those authorised in its technical datasheet. The objective of this study was to identify drugs recommended in rheumatology but considered for off-label use in Argentina.
MethodsA list of medications for certain selected rheumatic conditions was compiled. A drug was considered recommended if it was endorsed by a) at least one Argentine or Pan-American treatment guideline or consensus, or b) two international treatment guidelines, or c) one international treatment guideline and one selected textbook. Approval of these drugs for any condition in Argentina until December 31st, 2018 was explored, and medicines were divided into those with on-label indications and those considered for OL use.
ResultsOne hundred and thirty-six medications were analysed in 13 clinical conditions. Sixty-seven OL recommendations (49%) were found, and several drugs had more than one. All the conditions included the recommendation of at least 1 OL drug except osteoporosis and rheumatoid arthritis. The frequency of OL recommendations for the following conditions was 100%: calcium pyrophosphate dihydrate crystal deposition disease, polymyalgia rheumatica, Sjögren syndrome, and systemic sclerosis. The drugs with the highest number of OL recommendations were methotrexate (in 7 conditions), and glucocorticoids and mycophenolate (in 4). There were 2 OL recommendations for rituximab and 1 for abatacept.
ConclusionsAlmost all the rheumatic disorders analysed involved the recommendation of at least 1 OL medication, and in 4 conditions all the recommendations were OL. Most OL drugs recommended in rheumatology are neither biological nor small-molecule therapies.
El uso de fármacos al margen de las especificaciones (Off-label) es la prescripción de un fármaco para indicaciones diferentes a las autorizadas en su ficha técnica. El objetivo de este estudio fue identificar los medicamentos recomendados en reumatología, pero considerados al margen de las especificaciones en Argentina.
MétodosSe compiló un listado de medicaciones para determinadas situaciones reumáticas seleccionadas. Se consideró recomendado un fármaco si estaba respaldado por a) al menos una guía o consenso de tratamiento argentino o panamericano, b) por dos guías de tratamiento internacionales, o c) una guía de tratamiento internacional y un manual seleccionado. Se exploró la aprobación de dichos fármacos para cada situación en Argentina hasta el 31 de diciembre del 2018, dividiéndose los medicamentos en aquellos dentro de las especificaciones y los considerados al margen de estas.
ResultadosSe analizaron 136 fármacos de 13 situaciones clínicas. Se encontraron 67 recomendaciones al margen de las especificaciones (49%), y alguno de los medicamentos tenían más de una. Todas las situaciones incluyeron al menos un fármaco en estas condiciones, exceptuando osteoporosis y artritis reumatoide. La frecuencia de las recomendaciones al margen de las especificaciones fue del 100%: enfermedad de depósitos de cristales deshidratados de pirofosfato de calcio, polimialgia reumática, síndrome de Sjögren y esclerosis sistémica. Los fármacos con mayor número de estas recomendaciones fueron: metotrexato (en siete situaciones) y glucocorticoides y micofenolato (en cuatro). De igual manera, hubo dos para rituximab y una para abatacept.
ConclusionesCasi todos los trastornos reumáticos analizados implicaron la prescripción de, al menos, un fármaco con recomendaciones al margen de las especificaciones, y en cuatro situaciones todas fueron de este tipo. La mayoría de los fármacos sugeridos en reumatología pero al margen de las especificaciones no son terapias biológicas ni pequeñas moléculas.
Off-label (OL) drug use is the prescription of a drug for indications other than those authorised in its technical datasheet, and it is common practice in hospital settings.1
In Argentina, the National Administration of Drugs, Foods, and Medical Devices (ANMAT) is the institution that records, controls, and oversees the marketing authorisation for medicines in the country. The ANMAT, however, cannot prohibit OL drug use since the regulation of medical activity does not fall within its purview. Besides, professionals’ freedom to prescribe brings about innovation in clinical practice, especially when conventional therapies fail.2,3
It is well-known that drug manufacturing companies are bound to follow a rigorous procedure to demonstrate drug efficacy and safety. Nonetheless, OL drug use occurs on several grounds: a pharmaceutical company may desist from obtaining drug approval for a new condition due to the existence of a generic or commonly used medication or because of a rare condition. On the other hand, in the event of a terminal or life-threatening disease, or when the normal function of a vital organ is jeopardised, physicians are likely to use all available medicines based on their experience and logical reasoning, according to either the mechanism of action of the drugs or their efficacy verified in another ailment.4
Rheumatology prevents, diagnoses, and treats over 200 musculoskeletal disorders and systemic autoimmune diseases, and has experienced remarkable growth in recent decades.5 It encompasses a wide variety of low-prevalent, chronic, long-term debilitating conditions that seriously affect patients’ lives and “orphan diseases” for which there are frequently no treatment options and that do not arouse the pharmaceutical industry's interest.6
Nevertheless, health care payers may refuse to cover OL prescriptions. This could cause concern in patients who need affordable medicines with proven safety and efficacy.3,6
The following study was carried out with the aim of identifying drugs recommended in rheumatology but considered of OL use in Argentina.
The endorsement of a scientific society must not be mistaken for the approval of a regulatory authority.
The use of off-label medications is not forbidden, but it is strongly advisable to inform patients before prescribing a drug outside the terms of its marketing licence.
What this study adds – Take-home messagesMost of the rheumatic conditions studied herein include the recommendation of at least 1 off-label medication.
In most cases, at least 1 off-label medication was considered first-line therapy.
In everyday clinical practice, it is likely that a rheumatologist prescribes off-label medications since some of them are readily available and widely used.
A list of medications for certain selected adult rheumatic conditions was compiled by 3 of the authors (FV, CM y GS), and a thorough review of the undermentioned literature was subsequently carried out. The following sources were analysed for this purpose: (a) treatment guidelines and consensuses for rheumatic diseases developed and published by either Argentine or Pan-American scientific institutions until December 31st, 2018,7–14 (b) treatment guidelines and consensuses published by the American College of Rheumatology (ACR), the European League against Rheumatism (EULAR), and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)15–30 until December 31st, 2018 (when there was more than one version, the most recently updated recommendation was selected), and (c) the Kelley and Firestein's Textbook of Rheumatology, 10th edition, 2017.31
A drug was considered recommended if it was endorsed by (a) at least one Argentine or Pan-American treatment guideline or consensus, or (b) two international guidelines, or (c) one international guideline and the above-mentioned textbook.
Since there is no universally accepted definition of “recommended drugs” for adult rheumatic conditions, the authors arbitrarily determined that either a guideline written by peers of their nationality or two international prestigious sources would be enough evidence for a professional to consider that the medication is recommended for that condition.
Every medication recommended in the sources consulted was included regardless of the scientific validity of the information.
The choice of the abovementioned sources of information was made by consensus among the authors. Thus, the 13 rheumatic conditions were selected because national, Pan-American, and international guidelines on these diseases had been published prior to this study.
This study includes only systemic treatments (topical and intra-articular medications, and eye drops were excluded) for primary manifestations of the diseases (symptomatic treatments were excluded).
Glucocorticoids (GCs) were considered as a group and ranked as OL provided none of them had any approved indication for each disorder. If so much as one GC had an approved indication for a disorder, all of them were considered of on-label use for that disorder. The following GCs were analysed: meprednisone, prednisone, and methylprednisolone.
Nonsteroidal anti-inflammatory drugs (NSAIDs) were regarded as a group, and only in gout, calcium pyrophosphate dihydrate crystal deposition disease (CPPD), osteoarthritis, and ankylosing spondylitis. The NSAIDs evaluated in this study were ranked as OL with the same parameters applied for GCs. The following NSAIDs were assessed: celecoxib, diclofenac, etoricoxib, naproxen, indomethacin, and meloxicam.
As for fibromyalgia, only medicines recommended for pain relief were included.
From this initial list of medications, all the drugs with ANMAT marketing approval for any condition in Argentina until December 31st, 2018 were selected. Two researchers (FV and CM) reviewed the data on indications available in their patient information leaflets. Hence, medicines were divided into two groups: (a) those with on-label indications and (b) those considered of OL use in Argentina.
In a previous study published by our group,32 information inconsistencies in patient information leaflets of different registered trademarks of the same drug were observed (for example, original and generic drugs). Hence, in this study, when more than one registered trademark of the same medication was on the market with inconsistent indications in their patient information leaflets (namely, on-label for one and OL for another one), it was established that the medicine pertained to the group of on-label indication irrespective of the clinical considerations about the scientific evidence for the treatment.
In addition, both researchers (CM, FV) determined whether, in their judgement, the OL drugs were first- or second-line therapy in each condition. When they did not concur, a third researcher made the decision (GS). The correlation between the authors’ opinions evaluated through the Kappa Coefficient was: 0.44 (95% Confidence Interval 0.21–0.67).
Descriptive statistics were used for data analysisIn view of the fact that the data we analysed were in the public domain and did not include patients, no authorisation was requested from the Hospital Bioethics Committee, and informed consent was not required.
ResultsThirteen clinical conditions and 136 medications were included and are detailed in Table 1. Sixty-seven OL recommendations were observed (49%) although some medications had more than one.
Drugs recommended in rheumatology for 13 selected conditions, marketed in Argentina, and considered of either on label or of off label use.
| Condition | Consulted sources | Recommended, marketed, and considered of on label use in Argentina | Recommended, marketed, and considered of off label use in Argentina |
|---|---|---|---|
| RheumatoidArthritis | 7, 15, 16, 31 | Glucocorticoids, methotrexate, leflunomide, hydroxychloroquine, sulfasalazine, etanercept, infliximab, adalimumab, golimumab, certolizumab, rituximab, abatacept, tocilizumab, and tofacitinib. | None. |
| SystemicLupusErythematosus | 10, 13, 17, 31 | Glucocorticoids, azathioprine, belimumab chloroquine, cyclophosphamide and hydroxychloroquine, | First Line: methotrexate, mycophenolate.Others: abatacept, cyclosporine, gamma globulin, leflunomide, tacrolimus, and rituximab. |
| Psoriaticarthritis | 18, 19, 31 | Glucocorticoids, leflunomide, etanercept, infliximab, adalimumab, golimumab, certolizumab, ustekinumab, secukinumab, and apremilast. | First Line: methotrexate.1Others: sulfasalazine andcyclosporine.1 |
| Spondyloarthritis | 20, 21, 31 | NSAIDs, etanercept, infliximab, adalimumab, golimumab, certolizumab, ustekinumab, secukinumab, and apremilast. | First Line: none.Others: pamidronate and sulfasalazine. |
| Gout | 22, 23, 31 | NSAIDs, glucocorticoids, allopurinol, colchicine, febuxostat, and canakinumab. | First Line: none.Others: ACTH.2 |
| Osteoarthritis | 8, 24, 31 | NSAIDs, avocado and soybean unsaponifiables, diacerein, glucosamine, chondroitin sulfate, and duloxetine. | First Line: paracetamol. Others: hydroxychloroquine and tramadol. |
| Osteoporosis | 12, 25, 31 | Calcium, raloxifene, calcitonin, alendronate, risedronate, ibandronate, zoledronic acid, denosumab, vitamin D, strontium, teriparatide, and hormone replacement therapy. | None. |
| Fibromyalgia | 9, 26, 31 | Duloxetine, pregabalin, and milnacipran. | First Line: amitriptyline and tramadol.3Others: cyclobenzaprine and gabapentin. |
| SystemicSclerosis | 11, 27, 31 | None | First Line: glucocorticoids, nifedipine, bosentan, cilostazol, cyclophosphamide, methotrexate, and sildenafil.Others: alprostadil, ambrisentan, amlodipine, atorvastatin, azathioprine, diltiazem, tadalafil, penicillamine, epoprostenol, iloprost, mycophenolate, and treprostinil. |
| ANCA-associated Vasculitis | 28, 31 | Glucocorticoids, cyclophosphamide, and rituximab. | First Line: azathioprine, methotrexate, and mycophenolate.Others: leflunomide. |
| PolymyalgiaRheumatica | 29, 31 | None | First Line: glucocorticoids.Others: methotrexate. |
| Sjögren syndrome | 14, 31 | None | First Line: pilocarpine.Others: glucocorticoids, azathioprine, cyclophosphamide, cyclosporine, colchicine, dapsone, hydroxychloroquine, interferon alpha, gamma globulin, leflunomide, methotrexate, mycophenolate, sulfasalazine, and rituximab. |
| Calcium Pyrophosphate Dihydrate Crystal Deposition Disease (CPPD) | 30, 31 | None | First Line: NSAIDs.Others: ACTH,2 colchicine, glucocorticoids, hydroxychloroquine, and methotrexate. |
1 Approved for cutaneous psoriasis.
2 Adrenocorticotropic hormone (ACTH) is approved in the short term for rheumatic diseases in which corticosteroids are normally administered.
3 Approved for moderate-to-severe pain relief.
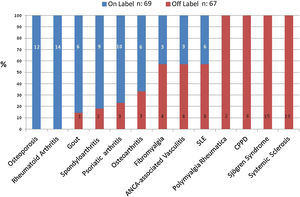
Except osteoporosis and rheumatoid arthritis, all the other conditions involved the recommendation of at least 1 OL medication, mostly as second-line therapy (see Fig. 1).
When we narrowed down the list to first-line therapy, 2 other conditions (spondyloarthritis, and gout) presented no OL recommendations.
Conversely, the frequency of OL recommendations in the following conditions was 100%: CPPD, polymyalgia rheumatica, Sjögren syndrome, and systemic sclerosis.
The following drugs had more than one OL recommendation: methotrexate (in 7 conditions), GCs (in 4 conditions), mycophenolate (in 4 conditions), cyclosporine, hydroxychloroquine, leflunomide, sulfasalazine, and azathioprine (in 3 conditions), and tramadol, cyclophosphamide, and adrenocorticotropic hormone (ACTH) (in 2 conditions). As to biological and small-molecule therapies, there were 2 OL recommendations for rituximab and one for abatacept.
DiscussionIn Argentina, except for osteoporosis and rheumatoid arthritis, there was at least one medication recommended OL in nearly all rheumatic disorders we analysed. In most cases, at least one OL medication was considered first-line therapy, and in 4 conditions all the recommendations were OL. Most of OL drugs recommended in rheumatology are neither biological nor small-molecule therapies.
OL drug use is characterised by the following features: (a) it is more common than thought, (b) although it is not authorised by the regulatory authorities, it is not forbidden either, (c) there is a certain degree of scientific evidence that supports it, (d) it is not without its risks, and (e) it is not exempt from legal problems.
According to a study performed in the United States of America and published in 2006, 21% of all the analysed prescriptions (that is, 150 million prescriptions) were OL. The study also pointed out a very high percentage of OL use of gabapentin (83%), amitriptyline, (81%) and rituximab (81%).33
A 2011 analysis of the Spanish Registry for Adverse Events of Biological Therapy in Rheumatic Diseases (BIOBADASER) highlighted that 11% of all the prescriptions of Anti-TNF drugs for rheumatic conditions were OL.34
A consensus study on OL drug use in Systemic Lupus Erythematosus (SLE) carried out by scientific authorities in Germany, Austria, and Switzerland, and published in 2012, admitted that even though knowledge on how to treat patients with SLE is constantly improving, not every patient with severe organic disease receives medical attention based on sufficient scientific evidence.35
In accordance with a study performed in Turkey and published in 2017, OL drug use generally reaches 21.5% in rheumatology, SLE is the most usual diagnosis (10.1%), and mycophenolate and rituximab are the most commonly prescribed drugs.36 Thus, in certain circumstances, a lot of patients benefit when they are administered drugs in scenarios other than the ones approved by the regulatory authorities.
A Canadian study published in 2012 underscored that OL drug use varied according to the attending physician and tended to be lower among professionals with evidence-based medical orientation since 79% of the prescriptions examined in this study lacked strong scientific evidence.37 Yet, OL drug use does not necessarily mean lack of scientific evidence: the physician may come across information about the use of a drug through continuing medical education programmes, posters presented in congresses of the speciality, or reports published in medical journals that even encourage the dissemination of this information.3 Therefore, treatment guidelines periodically published by the most respected scientific organisations (that have been the foundation of this analysis) include among their recommendations OL medications. Some scientific societies openly acknowledge this contradiction and plainly support OL drug use in common conditions and diseases, in special populations, or in disorders that are poorly defined.38
Lastly, there have been isolated cases of pharmaceutical companies that have been fined for advertising the OL use of some medications either directly to the population or indirectly through physicians.3,39
The existence of certain scientific evidence provides the attending physician with support for his demeanour, but it is imperative to grasp that OL drug use is not exempt from risks particularly in certain selected populations.
In a study of 5150 Spanish patients treated with Anti-TNF drugs, the occurrence of adverse effects was significantly associated with OL drug use.34 An Italian study published in 2016 analysed the OL use of IL-1 inhibitors and indicated that the adverse effects were considerably higher in patients older than 65 years of age in comparison with paediatric patients or adults between 15 and 65 years of age.40
Reports that mention the efficacy of OL drug use usually comprise only a few patients, and there may be certain publication bias as well, that is to say, only those cases in which the OL prescription has shown some efficacy are published and the rest are discarded.41
Finally, even though OL drug use is not forbidden, physicians must be aware that this practice is not without legal problems. OL drug use in one particular patient is not the same as its use in a clinical trial, but the attending physician is expected to clearly explain to the patient the level of scientific evidence that supports the use of the medication he is suggesting. Regardless of national regulations that may vary from one country to another, it would be good clinical practice to obtain the patient's written informed consent in the event of an OL prescription.6,42
This study has the following limitations: since a prescription audit has not been performed, neither the percentage of OL drug use in Argentina nor its economic cost has been estimated. In addition, audit studies may identify OL drug use with lower or even no scientific evidence at all. Still, in light of the results of the present study, it is likely that OL drug use in rheumatology in Argentina constitutes a significant proportion of all the medications prescribed within the speciality.
The compilation of a list of medications has been limited by the selection criteria arbitrarily created by the authors who acknowledge that there are other equally prestigious sources of information that have not been analysed. Consequently, the list of OL drugs currently used in rheumatology may be even longer.
The assertion that a recommended medication should be regarded as first-line therapy is based only on the authors’ opinion with a moderate level of agreement among them.
Although rheumatology encompasses hundreds of disorders, a limited number of diseases have been selected taking into account the most relevant within the speciality.
The indications set forth in patient information leaflets have been considered by disease. But this does not imply that it is recommended in any population with this disease: for example, a pregnant or lactating woman. Besides, given the multisystemic features of certain rheumatic disorders, medication approval for certain organ involvement (for example, kidney) does not mean approval for any other systemic involvement.
Among the strengths of the study, it is worth highlighting that we have made our utmost efforts to systematise the presentation of the drug information included in the present article, based on the most updated available scientific data.
In order to obtain drug approval in Argentina, ANMAT requests that it is authorised for human consumption in the domestic market of at least one of the countries mentioned in the Decree 150/92,43 (which includes Europe, the United States of America, Canada, Japan, and Israel). Among the sources we consulted, PANLAR, GRAPPA, EULAR, and ACR Treatment Guidelines have been included. Therefore, although the list of authorised medications may vary in different countries, some of the results and conclusions derived from this study may be extrapolated to other countries both in and out of the region.
In summary, the endorsement of a scientific society must not be mistaken for the approval of a regulatory authority, and it is strongly advisable to inform patients before prescribing a drug outside the terms of its marketing licence. According to the results of this study, it is highly likely that a rheumatologist prescribes OL medications in his daily practice since some of them are readily available and widely used.
Authors’ contributionFV, CM and GS, took part in the conception, design, data management and analysis of the study. All the authors wrote the article, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work.
Ethical approvalSince this article does not contain any studies with human participants performed by any of the authors, and the information analysed was in the public domain, authorisation from the Ethics Committee was not requested.
Consent for publication“Not applicable”.
Availability of data and materialsData sharing is not applicable to this article as no datasets were generated or analysed during the current study.
It has not been published or submitted for publication elsewhere.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that they have no conflict of interest.
The authors wish to thank Professor Ana Insausti for her cooperation in the translation of this research paper.
This study was presented as a poster at the 51st Argentine Congress of Rheumatology that took place in Mendoza, Argentina, 14–17 November 2018. (https://www.revistasar.org.ar/revistas.php).