To determine the disease burden and costs in patients with hip or knee OA and chronic moderate-to-severe refractory pain, receiving strong opioids in Spain.
Materials and methodsThis was a 36-month longitudinal secondary analysis of the real-word OPIOIDS study. Patients aged ≥18 years with hip or knee OA and chronic moderate-to-severe refractory pain receiving strong opioids were considered. The disease burden included analgesia assessments (NRS scale), cognitive functioning (MMSE scale), basic activities of daily living (Barthel index), and comorbidities (severity and frequency). Costs due to the use of healthcare resources and productivity loss were estimated.
Results2832 patients were analyzed; age was 72.0 years (SD=14.3), 76.8% were women. Patients had mainly been treated with fentanyl (n=979; 37.6%), tapentadol (n=625; 24.0%), oxycodone (n=572; 22.0%), and buprenorphine (n=425; 16.3%). Pain intensity decreased by 1 point (13.7%), with a 2.6-point decline in the cognitive scale (14.3%, with a 5.3%-increase in patients with cognitive deficit) over a mean treatment period of 384.6 days (SD: 378.8). Barthel scores decreased significantly yielding to a slightly increase in proportion of patients with severe-to-total dependency; 1.2%–2.9%. In the first year of treatment, average healthcare costs were €2013/patient, whereas the average productivity loss cost was €12,227/working-active patient.
Discussion and conclusionsStrong opioids resulted in high healthcare costs with a limited reduction in pain, an increase in cognitive deficit, and a slight increase of patients with severe to total dependency over 36 months of treatment.
Determinar la carga de la enfermedad y los costes en pacientes con osteoartritis de cadera y rodilla y dolor crónico refractario moderado-severo, en tratamiento con opioides mayores en España.
Materiales y métodosSe trata de un subanálisis de 36 meses de duración, procedente del estudio observacional OPIOIDS. Participaron pacientes con una edad ≥18 años, diagnosticados con osteoartritis de cadera y rodilla y dolor crónico refractario moderado-severo, en tratamiento con opioides mayores. La carga de la enfermedad incluyó la evaluación de la analgesia (escala NRS), del funcionamiento cognitivo (escala MMSE), de la capacidad para realizar las actividades de la vida diaria (índice de Barthel) y de las comorbilidades (gravedad y frecuencia). También se estimaron los costes asociados al uso de recursos sanitarios y a la productividad laboral.
ResultadosSe analizaron 2.832 pacientes (edad: 72,0 años [DE: 14,3]; mujeres: 76,8%), que habían sido principalmente tratados con fentanilo (n=979; 37,6%), tapentadol (n=625; 24,0%), oxicodona (n=572; 22,0%) y buprenorfina (n=425; 16,3%). La intensidad del dolor disminuyó una unidad (13,7%), con una reducción de 2,6 unidades en la escala cognitiva (14,3% y aumento del 5,3% en los pacientes con déficit cognitivo) durante una media de 384,6 días (DE: 378,8). Las puntuaciones en la escala de Barthel disminuyeron significativamente, con un ligero aumento en la proporción de pacientes con dependencia grave/total, entre 1,2% y 2,9%. En el primer año, los costes sanitarios medios fueron 2.013€/paciente, mientras que los costes medios de pérdida de productividad fueron 12.227€/trabajador.
Discusión y conclusionesEl tratamiento con opioides mayores durante 36 meses implicó elevados costes sanitarios, con una eficacia analgésica limitada, un aumento del déficit cognitivo y un ligero aumento de los pacientes con dependencia grave/total.
Osteoarthritis (OA) is a long-term chronic disease that affects up to 29.4% of the Spanish population over 40 years of age.1 The prevalence of OA in hip and knee was estimated at 5.13% and 13.83%, respectively.1 Between 6% and 24% of patients with OA have chronic pain (>3 months in duration), and the incidence increases with age.2 OA also has a high impact on patients’ quality of life3 and is one of the main causes of absenteeism at work.4
Opioids are prescribed for the treatment of moderate-to-severe pain in patients with an insufficient response to non-narcotic analgesics and/or nonsteroidal anti-inflammatory drugs (NSAIDs). However, their effectiveness for OA pain is limited and use of opioids is linked to adverse effects such as cognitive decline and drug dependency, which may lead to discontinuation.5–7 A recent meta-analysis showed that 12-week treatment with opioids provided minimal relief of OA symptoms and may cause discomfort in most patients.8 Around 11%–15% of patients with moderate-to-severe pain may be resistant to NSAIDs and weak opioids,9,10 and this refractory pain may require the administration of strong opioids such as morphine, oxycodone, fentanyl, buprenorphine, or tapentadol.11 However, due to limited efficacy and safety concerns, opioid use for OA is not recommended by either the Osteoarthritis Research Society International (OARSI) or the American College of Rheumatology (ACR).12,13
In Spain, the OPIOIDS study (Outcomes in Patients usIng Opioids In Painful Disorders in Spain) analyzed the clinical outcomes, healthcare resource use, and costs associated with the management of patients with OA and chronic nociceptive pain who initiated treatment with an opioid, according to real-world practice.2 A secondary analysis estimated the disease burden and costs in patients with OA and chronic moderate-to-severe pain refractory to NSAIDs+opioids (sequentially or concomitantly).10 However, there is a lack of information about the overall disease burden in patients with hip or knee OA. Therefore, this study aimed to estimate the burden and costs associated with the management of these patients being treated with strong opioids in Spain.
Material and methodsDesign, site, and data sourceThis is a secondary analysis of the OPIOIDS study, which was a non-interventional, longitudinal, retrospective study whose methodology has been previously published.2,10
Study populationThe study population was obtained from electronic medical records (EMRs) in the BIG-PAC® anonymized database and complementary financing databases from 7 Spanish Autonomous Communities.
The recruitment period was between January 1, 2010, to December 31, 2015, and patients were followed from the index date up to a maximum of 36 months and/or until treatment discontinuation (follow-up period). The index date was defined as the start of NSAID+opioid treatment during the recruitment period. The overall cohort included patients with chronic moderate-to-severe pain due to hip or knee OA who were refractory to an analgesic therapy consisting of a combination of an NSAID+opioid (administered sequentially or concomitantly). Patients could also be taking paracetamol (acetaminophen) or metamizole. This secondary analysis included the subset of patients who were taking a strong opioid. The OA diagnosis was defined according to the International Classification of Diseases, 10th Edition, Clinical Modification (ICD-10-CM) coding system (hip [M16] and knee [M17]), and chronic pain was defined as pain persisting for >3 months.9 Patients were determined to be refractory to treatment if after receiving analgesic therapy they still scored >5 (moderate-to-severe pain) on a numeric rating scale (NRS) of 11 points (0 no pain, 10 worst possible pain).14
The inclusion criteria were age ≥18 years and diagnosis of OA with chronic pain of more than 3 months’ duration. Patients were also required to have a minimum of 2 EMRs in the database at least 12 months before the index date (i.e., active patients), which means that they had received ≥2 prescriptions of an NSAID alone or combined with another non-narcotic analgesic, such as metamizole or paracetamol. The study also included patients in the chronic prescription program (with a record of the daily dose and duration of each treatment administered), and those with a regular follow-up (≥2 EMRs from the index date).
The full exclusion criteria were previously published.2 Of note, patients who discontinued any of the index treatments because of tolerability problems (defined as >30 days without renewing the initial medication dispensed at the community pharmacy and without renewals during the study follow-up) and those who received a prescription from their physician but did not have it filled by a pharmacy (primary failure of therapeutic adherence) were excluded from analyses.
Treatment description, adherence, and persistenceDrug treatments were described using the Anatomical Chemical Therapeutic Classification System (ATC; N02AA01 to N02AX06). All prescribed drugs were at the discretion of the physician. We included (a) non-opioid analgesics (NSAIDs, paracetamol, metamizole), (b) weak opioids (codeine, dihydrocodeine, tramadol [alone or in combination], dextropropoxyphene), and (c) strong opioids (buprenorphine, fentanyl, hydromorphone, morphine, oxycodone plus naloxone, oxycodone, pethidine, tapentadol). Prescription records were obtained for the 12 months before and 36 months after the index date.
Adherence, medication possession ratio (MPR), and persistence were estimated following the same methodology as in the OPIOIDS study.2 Treatment discontinuation was defined as a >30-day period without refilling the latest opioid prescription in those patients who have been dispensed >2 prescriptions of the same opioid during the study period. Refractory patients could discontinue the study by (a) switching to an analgesic other than those previously used, (b) referral to the pain unit or surgery for invasive treatments, (c) loss to follow-up, and/or (d) death from any cause.
Disease burdenWe collected data regarding (a) the change in pain intensity using the NRS,14 (b) functional variations in the basic activities of daily living (BADL) using the Barthel index15 (patients aged ≥65 years), and (c) cognitive changes using the Mini-Mental State Examination (MMSE) scale16 (patients aged ≥65 years) between the nearest date before the index date and the end of the study. The scales were used in their validated Spanish versions and the absolute change in their natural and relative units was calculated as the percentage change from baseline. For BADL, the criterion of the interpretation of Barthel's scale was followed, considering relevant dependency as a severe-to-total limitation on functionality (limit corrected by covariates no greater than 60 points).15 For cognitive functioning, a score on the MMSE of <20 was interpreted as a moderate-to-severe cognitive deterioration (cognitive deficit).16
Comorbidities were recorded at the index date (using ICD-10-CM). The Charlson Comorbidity Index (CCI) was used to summarize the health status and to estimate the comorbidity severity for each patient.17 In addition, all-cause deaths were recorded during the study period.
Disease costThe societal and Spanish National Health System (SNHS) perspectives were considered to calculate healthcare and non-healthcare (indirect) costs. Healthcare costs were those relating to medical visits, hospitalizations, diagnostic/therapeutic tests, and drugs, whereas non-healthcare costs were those relating to lost productivity.
Costs were expressed in 2018 Euros. Healthcare costs were calculated by multiplying the unit cost (Online Resource 1) by the frequency of use during the follow-up. Drug costs were quantified using the retail price per pack when they were dispensed from the community pharmacy.18 To estimate the productivity loss (non-healthcare costs), the number of days of work disability were considered in combination with the mean salary for the Spanish population19 (Online Resource 1).
Statistical analysisData were validated and reviewed using exploratory analyses. Registration or coding errors were also analyzed. The representativeness of the database in comparison with the Spanish population was estimated in a previous study.20
A descriptive univariate statistical analysis was performed. Absolute and relative frequencies were calculated for qualitative data, whereas quantitative data were expressed using means, standard deviations (SDs), medians, and the 25th and 75th percentiles (interquartile ranges [IQRs]). 95% confidence intervals (CIs) were used to estimate the parameters, considering the total number of patients with non-missing values. Statistical tests were used for paired groups (means, proportions). The before–after differences were shown with the 95% CI of the difference calculated by non-parametric resampling (1000 bootstrap iterations). An adjusted univariate linear model was used to compare healthcare costs when independent groups were compared. Covariates included were sex, age, comorbidities (number and CCI), and time from diagnosis. The Bonferroni correction was applied in case of multiple comparisons. Statistical analysis was completed using IBM SPSS, version 23.0 (NY, USA).
Compliance with ethicsThis study was carried out in line with the Declaration of Helsinki of 1964, and its later amendments. Patient consent was not obtained, since Spanish legislation excludes data that is aggregated for analysis. Personal data were de-identified as specified in the Spanish Law 15/1999, of 13 December, on Personal Data Protection, and the Organic Law 3/2018 of December 5, On the Protection of Personal Data and Guarantee of Digital Rights. The study was approved by the Research Ethics Committee of the Hospital de Terrassa, Barcelona, Spain (Code: PFI-OP-2018-01) on March 11, 2019.
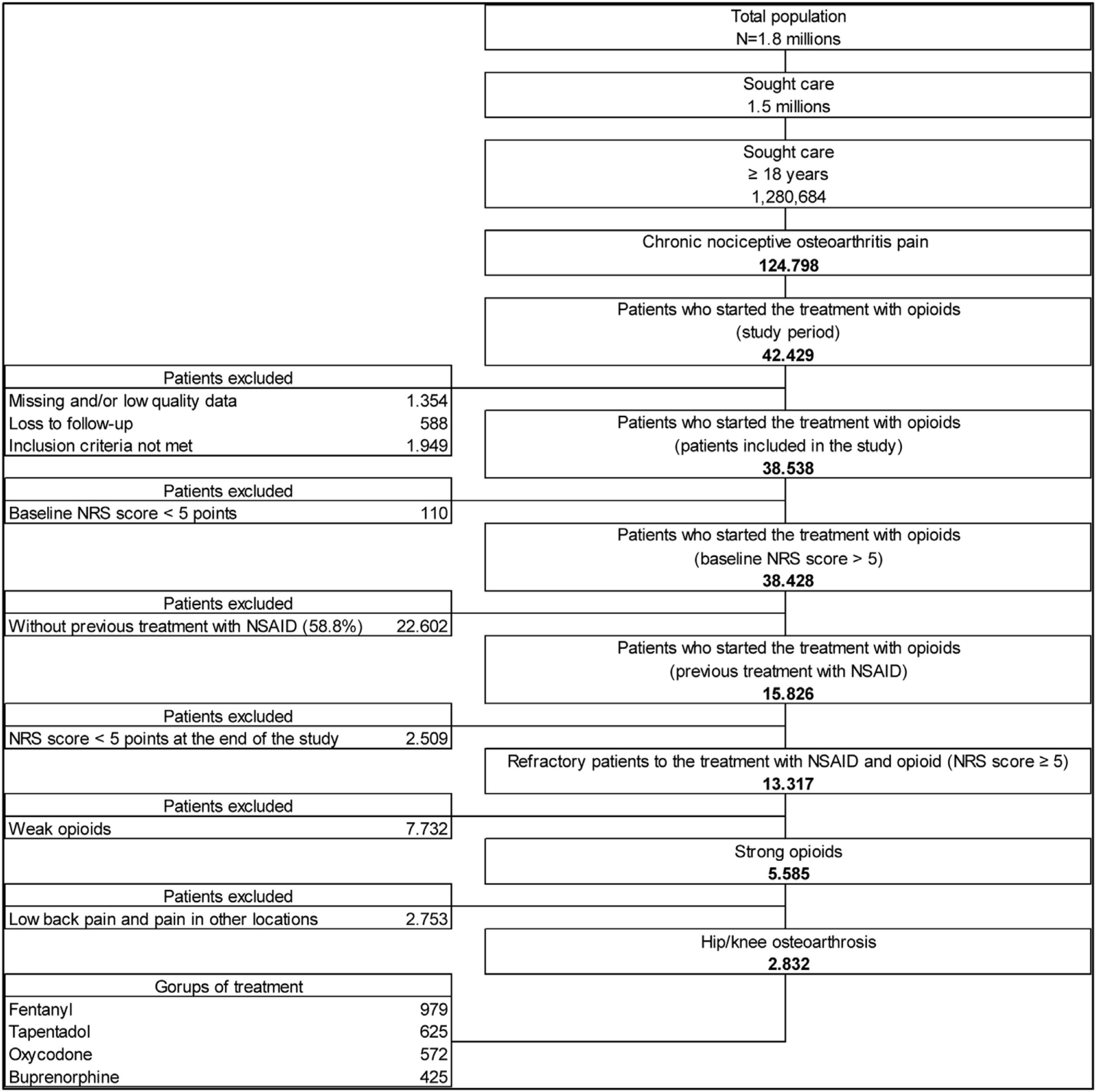
ResultsThe study found 13,317 patients refractory to analgesic treatment with an NSAID plus opioid: 58.1% had received an NSAID plus weak opioid and 41.9% had been treated with an NSAID plus a strong opioid. It was estimated that 50.7% (n=2832) suffered hip and/or knee OA and met the criteria for inclusion in this secondary analysis of the OPIOIDS study. Our results showed that these patients had mainly been treated with fentanyl (n=979; 37.6%), tapentadol (n=625; 24.0%), oxycodone (n=572; 22.0%), or buprenorphine (n=425; 16.3%) (Fig. 1). It was estimated that 231 patients were on treatment with other strong opioids: 129 patients received morphine, 84 patients hydromorphone, and 18 patients other strong opioids. Of those on treatment with fentanyl, most (89.6%) received a transdermal patch formulation. Patients prescribed oxycodone included those receiving a combination of oxycodone+naloxone (85.7%) or oxycodone alone (14.3%).
The mean (SD) age of the study population was 72.0 (14.3) years and 76.8% were female (Table 1). The overall study population had a mean (SD) of 3 (1.8) diagnoses. The mean (SD) CCI was 1.9 (1.8); a third of the study population (33.3%) had a mean score ≥3. Overall, the most common comorbidities were hypertension (55.9%), dyslipidemia (55.1%), and osteoporosis (33.3%) (Table 1).
Baseline characteristics and burden of comorbidity in all patients according to type of strong opioid.
| Study groups | Fentanyl | Tapentadol | Oxycodonea | Buprenorphine | p | Total |
|---|---|---|---|---|---|---|
| Number of patients | (n=979) | (n=625) | (n=572) | (n=425) | (n=2832)b | |
| Sociodemographic features | ||||||
| Mean (SD) age, years | 70.1 (12.1) | 67.1 (14.3) | 71 (13.7) | 73.9 (13.9) | <0.001 | 72 (14.3) |
| 18–44 years | 3.8% | 7.4% | 3.5% | 4.0% | <0.001 | 4.2% |
| 45–64 years | 26.6% | 36.0% | 29.5% | 20.0% | <0.001 | 26.3% |
| 65–74 years | 30.4% | 23.8% | 27.1% | 25.9% | <0.001 | 23.1% |
| ≥75 years | 39.2% | 32.8% | 39.9% | 50.1% | <0.001 | 46.4% |
| Sex (female) | 75.9% | 73.0% | 72.9% | 84.2% | <0.001 | 76.8% |
| General comorbidity | ||||||
| Mean (SD) diagnoses | 3.1 (1.9) | 2.7 (1.7) | 3 (1.8) | 2.9 (1.7) | <0.001 | 3 (1.8) |
| 4+ | 41.8% | 28.2% | 33.6% | 34.8% | 35.5% | |
| Mean (SD) Charlson Comorbidity Index | 1.9 (1.9) | 1.6 (1.8) | 1.9 (1.7) | 1.8 (1.8) | <0.001 | 1.9 (1.8) |
| 0 | 24.1% | 36.0% | 25.7% | 25.6% | <0.001 | 26.2% |
| 1 | 27.8% | 23.8% | 27.4% | 28.7% | <0.001 | 25.4% |
| 2 | 17.7% | 11.8% | 13.1% | 14.8% | <0.001 | 15.0% |
| 3+ | 30.4% | 28.3% | 33.7% | 30.8% | <0.001 | 33.3% |
| Associated comorbidities | ||||||
| High blood pressure | 54.4% | 49.0% | 57.9% | 55.5% | 0.002 | 55.9% |
| Diabetes | 21.5% | 23.2% | 24.7% | 26.6% | 0.384 | 25.5% |
| Dyslipidemia | 54.4% | 51.0% | 54.4% | 56.2% | 0.008 | 51.1% |
| Obesity (BMI ≥30kg/m2) | 17.7% | 24.0% | 24.7% | 24.7% | 0.747 | 24.1% |
| Ischemic heart disease | 16.5% | 8.2% | 8.4% | 8.0% | 0.001 | 10.2% |
| Cerebrovascular accident | 12.7% | 6.9% | 9.8% | 8.7% | 0.148 | 9.2% |
| Heart failure | 8.9% | 6.7% | 9.8% | 10.6% | <0.001 | 11.4% |
| Kidney failure | 7.6% | 4.5% | 6.1% | 6.4% | 0.004 | 7.1% |
| Asthma | 10.1% | 10.2% | 11.7% | 10.4% | 0.758 | 11.3% |
| COPD | 10.1% | 9.0% | 8.9% | 6.6% | 0.161 | 9.3% |
| Dementia | 13.9% | 5.6% | 7.3% | 9.4% | <0.001 | 10.2% |
| Depressive syndrome | 27.8% | 20.5% | 24.3% | 19.5% | 0.026 | 23.4% |
| Malignancies | 5.1% | 5.3% | 6.5% | 5.2% | 0.892 | 5.7% |
| Osteoporosis | 35.4% | 28.3% | 32.7% | 34.4% | 0.031 | 33.3% |
| Metabolic syndrome | 29.2% | 26.7% | 29.5% | 28.5% | <0.001 | 30.6% |
| Additions | ||||||
| Active smokers (daily) | 18% | 14% | 12% | 11% | 0.090 | 12% |
| Alcohol consumption ≥30 grams/day | 1.6% | 1.8% | 1.9% | 1.7% | 0.397 | 1.7% |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; SD: standard deviation.
The median (IQR) time from diagnosis was 0.54 (0.30–1.4) years. The opioid treatment lasted a median (IQR) of 181 (70–703) days (Table 2). The treatment adherence in the overall population was 32.5% (95% CI, 30.7%–34.2%) after 12 months and 16.5% (95% CI, 15.1%–17.9%) after 36 months, without notable differences among the 4 groups (p=0.633 and p=0.632, respectively) (Table 2).
Treatment persistence and adherence, medication possession ratio and deaths by group (during the follow-up period).
| Study groups | Fentanyl | Tapentadol | Oxycodonea | Buprenorphine | p | Total |
|---|---|---|---|---|---|---|
| Number of patients | (n=979) | (n=625) | (n=572) | (n=425) | (n=2832) | |
| Time from diagnosis, years | 1.0 (0.9) | 1.2 (1.1) | 1.1 (1.0) | 1.2 (1.3) | <0.001 | 1.2 (1.2) |
| Median (IQR) | 0.29 (0.26–0.41) | 0.86 (0.35–2.06) | 0.60 (0.31–1.33) | 0.56 (0.30–1.29) | <0.001 | 0.54 (0.30–1.40) |
| Duration of treatment, days | 378.7 (365.8) | 386.4 (374.3) | 383.6 (377.8) | 385.9 (372.5) | <0.001 | 384.6 (378.8) |
| Median (IQR) | 192 (73–703) | 186 (72–695) | 179 (70–691) | 188 (72–710) | 181 (70–703) | |
| Medication possession ratio | ||||||
| Percentage | 71.6% | 75.0% | 72.5% | 73.6% | 0.513 | 73.9% |
| 95% CI | 61.7%–81.5% | 71.6%–78.4% | 68.8%–76.2% | 69.4%–77.8% | 72.3%–75.5% | |
| Treatment adherence (%) | ||||||
| 12 months | 31.5% | 34.3% | 31.6% | 32.4% | 0.633 | 32.5% |
| 95% CI | 21.3%–41.7% | 30.6%–38% | 27.8%–35.4% | 28.0%–36.8% | 30.7%–34.2% | |
| 36 months | 15.1% | 17.2% | 15.9% | 17.3% | 0.632 | 16.5% |
| 95% CI | 7.2%–23% | 14.2%–20.2% | 12.9%–18.9% | 13.7%–20.9% | 15.1%–17.9% | |
| Discontinuation of strong opioid throughout 36 months of follow-up (%) | ||||||
| Poor tolerabilityb | 7.9% | 7.6% | 8.2% | 7.3% | 0.631 | 8.1% |
| 95% CI | 2%–13.8% | 5.5%–9.7% | 6.0%–10.4% | 4.8%–9.8% | 7.1%–9.1% | |
| Poor responsec | 45.6% | 43.9% | 45.0% | 45.2% | 0.774 | 45.0% |
| 95% CI | 34.6%–56.6% | 40%–47.8% | 40.9%–49.1% | 40.5%–49.9% | 43.2%–46.8% | |
| Other changesd | 27.4% | 27.0% | 26.1% | 26.3% | 0.806 | 26.0% |
| 95% CI | 17.6%–37.2% | 23.5%–30.5% | 22.5%–29.7% | 22.1%–30.5% | 24.4%–27.6% | |
| Deaths any cause | 4.0% | 4.3% | 4.8% | 3.9% | 0.494 | 4.4% |
| 95% CI | 0.3%–8.3% | 2.7%–5.9% | 3.0%–6.6% | 2.1%–5.7% | 3.6%–5.2% | |
CI: confidence interval; IQR: interquartile range (25th percentage–75th percentage).
Values expressed as a percentage or mean (standard deviation), CI.
During the 12 months before the index date, patients were taking an average (SD) of 2 (0.7) medications. All patients were on treatment with an NSAID, and most (77.0%) also received a weak opioid. In the first year after the index date, all patients reduced the mean (SD) number of medications to 1.7 (0.7), mainly due to weak opioids being discontinued when initiating the strong opioid therapy. During the 36-month follow-up, the mean (SD) number of prescriptions per patient increased to 2.0 (0.8).
In general, 63% of medications were prescribed in family medicine settings, whereas around 19.3% were prescribed in setting related to anesthesia and resuscitation (Table 3).
Analgesic medication in the total sample and according to type of opioid.
| Study groups | Fentanyl | Tapentadol | Oxycodonea | Buprenorphine | p | Total |
|---|---|---|---|---|---|---|
| Number of patients | (n=979) | (n=625) | (n=572) | (n=425) | (n=2832) | |
| 12 months pre-opioid | ||||||
| NSAID | 100.0% | 100.0% | 100.0% | 100.0% | – | 100.0% |
| Paracetamol | 53.2% | 54.7% | 59.6% | 60.7% | <0.001 | 61.6% |
| Metamizole | 32.9% | 34.4% | 33.6% | 39.3% | 0.207 | 36.3% |
| Weak opioid | 84.0% | 72.4% | 73.6% | 76.6% | <0.001 | 77.0% |
| Mean number of medications (SD) | 1.9 (0.7) | 1.9 (0.7) | 1.9 (0.7) | 2 (0.7) | <0.001 | 2 (0.7) |
| 12 months post-strong opioid initiation | ||||||
| NSAID | 84.8%‡ | 74.88%‡ | 72.4%‡ | 76.0%‡ | 0.001 | 72.4%‡ |
| Paracetamol | 55.7% | 61.8%† | 67.3%† | 66.6% | 0.001 | 66.9%‡ |
| Metamizole | 39.2% | 28.2%† | 27.6% | 34.8% | 0.010 | 31.4%‡ |
| Mean number of medications (SD) | 1.8 (0.7) | 1.6 (0.7)‡ | 1.7 (0.7)‡ | 1.8 (0.8)‡ | 0.023 | 1.7 (0.7)‡ |
| 36 months post-strong opioid initiation | ||||||
| NSAID | 89.9% | 84.6% | 84.1% | 85.6% | 0.208 | 83.8% |
| Paracetamol | 68.4% | 68.6% | 73.4% | 72.0% | 0.001 | 73.7% |
| Metamizole | 57.0% | 42.9% | 45.6% | 49.4% | 0.050 | 46.9% |
| Mean number of medications (SD) | 2.2 (0.7) | 2 (0.8) | 2 (0.8) | 2.1 (0.9) | 0.033 | 2 (0.8) |
| Prescribing medical specialty (total opioids) | ||||||
| Family medicine | 77.2% | 48.5% | 51.4% | 68.9% | <0.001 | 63.0% |
| Trauma | 1.3% | 9.1% | 4.5% | 2.1% | <0.001 | 4.0% |
| Anesthesia and resuscitation | 2.5% | 30.2% | 31.5% | 15.3% | <0.001 | 19.3% |
| Rheumatology | 8.9% | 1.3% | 2.6% | 1.6% | <0.001 | 2.0% |
| Rehabilitation | 0.0% | 4.5% | 1.4% | 1.6% | <0.001 | 1.6% |
| Other specialties | 10.1% | 6.4% | 8.6% | 10.4% | <0.001 | 10.1% |
NSAID: nonsteroidal anti-inflammatory drug; SD: standard deviation.
Values expressed as percentage or mean (SD).
The mean (SD) pain severity score at the index date was 8.3 (0.9), which decreased at the end of the follow-up period to 7.3 (0.9) (p<0.001). Therefore, patients showed a 1-point decrease (13.7%) in NRS score. It was estimated that 2.4% of patients had a reduction ≥50% (Table 4).
Change in pain severity, cognitive functioning, and disability at the end of follow up.
| Study groups | Fentanyl | Tapentadol | Oxycodonea | Buprenorphine | p | Total |
|---|---|---|---|---|---|---|
| Number of patients | (n=979) | (n=625) | (n=572) | (n=425) | (n=2832) | |
| Pain severity (11-point NRS) | ||||||
| Initial | 8.2 (0.9) | 8.4 (0.9) | 8.2 (1) | 8.3 (0.9) | 0.014 | 8.3 (0.9) |
| Final | 7.4 (0.8)‡ | 7.2 (0.9)‡ | 7.2 (0.9)‡ | 7.3 (0.9)‡ | 0.308 | 7.3 (0.9)‡ |
| Difference (absolute) | −0.8 | −1.2 | −1.0 | −1.0 | 0.467 | −1.0 |
| 95% CI | −1.0, −0.5 | −1.3, −1.1 | −1.1, −0.9 | −1.1, −0.9 | −1.1, −1.0 | |
| Difference (relative, %) | −10.8% | −16.7% | −13.9% | −13.7% | 0.171 | −13.7% |
| Responders (%) | ||||||
| Pain reduction≥30% | 13.1% | 14.6% | 14.9% | 12.7% | 0.322 | 13.6% |
| Pain reduction≥50% | 2.6% | 2.7% | 2.6% | 2.1% | 0.887 | 2.4% |
| Cognitive function (MMSE) | ||||||
| Initial | 24.4 (5.7) | 25.1 (5.8) | 24.5 (5.9) | 25.3 (5) | 0.002 | 24.5 (5.9) |
| Final | 21.8 (5.7)* | 22.3 (5.8)‡ | 21.9 (5.9)‡ | 22.7 (5)‡ | 0.003 | 21.9 (5.9)‡ |
| Absolute difference | −2.6 | −2.8 | −2.6 | −2.6 | 0.597 | −2.6 |
| 95% CI | −2.6, −2.6 | −2.6, −2.6 | −2.6, −2.6 | −2.6, −2.6 | −2.6, −2.6 | |
| Relative difference | −14.3% | −13.6% | −14.3% | −13.6% | 0.727 | −14.3% |
| Patients with cognitive deficit (MMSE<20 points) | ||||||
| Initial | 19.0% | 16.5% | 15.3% | 13.4% | 0.191 | 15.7% |
| Final | 21.1% | 19.1% | 19.2% | 17.2% | 0.047 | 21%‡ |
| Difference | 2.1% | 2.6% | 3.9% | 3.8% | 0.426 | 5.3% |
| Disability in BADL (Barthel) | ||||||
| Initial | 61.8 (15.3) | 62.3 (13.3) | 61.7 (13.6) | 61.7 (16) | 0.181 | 61.9 (14.8) |
| Final | 60.7 (16.6) | 61.2 (22.5) | 60.8 (24) | 60.7 (23.4) | 0.419 | 60.9 (22.8) |
| Difference (absolute) | −1.1 | −1.1 | −0.9 | −1.0 | 0.647 | −1.0 |
| 95% CI | −1.3, −0.9 | −1.2, −1.1 | −1.0, −0.8 | −1.1, −0.9 | −1.0, −0.9 | |
| Difference (relative) | −1.7% | −1.6% | −1.7% | −1.7% | 0.887 | −1.6% |
| Patients with severe to total dependence (Barthel≤60 points) | ||||||
| Initial | 18.8% | 19.7% | 24.0% | 22.4% | 0.323 | 22.1% |
| Final | 21.7% | 21.2% | 26.1% | 23.6% | 0.055 | 23.9% |
| Difference | 2.9% | 1.5% | 2.1% | 1.2% | 0.248 | 1.8% |
BADL: Basic Activities of Daily Living; CI: confidence interval; MMSE: Mini-Mental State Examination; NRS: numeric rating scale; SD: standard deviation.
Values expressed as percentage or mean (SD or 95% CI). Pain severity measured with a numeric rating scale (NRS) of 11 points (0 no pain, 10 worst possible pain). Cognitive function determined with the MMSE test, establishing cognitive deficit for scores <20. Functional variations in BADL assessed by Barthel's test, with severe-to-total disability (dependence) for scores ≤60 points.
Cognitive function, measured with the MMSE scale, showed an average (SD) score at index of 24.5 (5.9). At the end of the follow-up period, patients had an average (SD) score of 21.9 (5.9), showing a decrease in cognitive function of 2.6 points (14.3%). The prevalence of the cognitive deficit (MMSE<20 points) increased by 5.3%, from 15.7% to 21.0% (p<0.001) during the follow-up period (Table 4).
The average (SD) score in the Barthel scale was similar at the index date (61.9 [14.8] points) and at the end of the follow-up period (60.9 [22.8] points; difference: 1.0 point [1.6%]). No difference was seen among the 4 treatment groups (p=0.181 and p=0.419, respectively). However, Barthel scores decreased slightly (but statistically significantly) at the end of treatment, with each of the opioids analyzed yielding to an increment in the percentage of patients with severe-to-total dependence (Barthel index ≤60 points) between 1.2% and 2.9% at the end of follow-up (Table 4).
Disease costsHealthcare costsThe healthcare costs associated with the management of patients with chronic moderate-to-severe pain due to hip or knee OA during the 12 months before the index date amounted to an annual average cost of €2013 (95% CI, €1920–€2106) per patient (Table 5). The healthcare annual costs per patient during the first year after the index date were higher than those registered in the year before the index date (€2013 vs. €2742; p<0.001). The cost of the analgesic treatment per patient was at least 5 times higher in comparison to the cost in the 12 months before the index date (€678 vs. €132, respectively; p<0.001). The most expensive alternative was fentanyl (€805 [95% CI, €742–€868]) (Table 5).
Health costs in Euros per patient and per day in the total sample and per group.
| Study groups | Fentanyl | Tapentadol | Oxycodonea | Buprenorphine | p | Total |
|---|---|---|---|---|---|---|
| Number of patients | (n=979) | (n=625) | (n=572) | (n=425) | (n=2832) | |
| 12 months pre-opioid | ||||||
| Total analgesics cost | ||||||
| Per patient | 160 (149–171) | 133 (124–142) | 144 (134–153) | 125 (115–135) | 0.003 | 132 (127–136) |
| Per day | 0.86 (0.64–1.08) | 0.72 (0.62–0.82) | 0.83 (0.71–0.89) | 0.69 (0.62–0.76) | 0.72 (0.68–0.76) | |
| Opioid analgesia cost | ||||||
| Per patient | 116 (108–124) | 119 (106–132) | 121 (107–135) | 115 (99–131) | 116 (110–122) | |
| Per day | 0.47 (0.30–0.64) | 0.48 (0.42–0.54) | 0.49 (0.43–0.55) | 0.46 (0.40–0.52) | 0.47 (0.45–0.49) | |
| Healthcare cost excluding analgesia | 1054 (959–1149) | 2204 (2007–2401) | 1971 (1765–2177) | 1602 (1363–1841) | 1881 (1788–1974) | |
| Total healthcare cost | 1214 (1130–1298) | 2337 (2140–2534) | 2115 (1909–2321) | 1727 (1488–1966) | 2013 (1920–2106) | |
| 12 months post-strong opioid initiation | ||||||
| Total analgesics cost | ||||||
| Per patient | 805 (742–868)‡ | 795 (688–902)‡ | 581 (542–621)‡ | 436 (419–453)‡ | 678 (652–705)‡ | |
| Per day | 7.70 (5.48–9.92)‡ | 7.5 (6.5–8.5)‡ | 5.63 (5.08–6.18)‡ | 4.20 (3.64–4.76)‡ | <0.001 | 6.70 (6.30–7.10)‡ |
| Opioid analgesia cost | ||||||
| Per patient | 708 (611–804) | 751 (737–765) | 509 (470–548) | 375 (360–390) | <0.001 | 614 (588–640) |
| Per day | 5.3 (4.7–5.8) | 5.8 (5.0–6.6) | 4.3 (3.6–5.1) | 2.8 (2.5–3.2) | <0.001 | 5.1 (4.7–5.5) |
| Healthcare cost excluding analgesia | 1802 (1728–1876)‡ | 2540 (2199–2882)* | 2323 (1977–2670)† | 1497 (1210–1785) | <0.001 | 2064 (1919–2208)† |
| Total healthcare cost | 2607 (2434–2780)‡ | 3335 (2887–3784)‡ | 2905 (2551–3258)‡ | 1934 (1645–2223) | <0.001 | 2742 (2594–2890)‡ |
| 36 months follow-up | ||||||
| Total analgesics cost | ||||||
| per patient | 2034 (1903–2165) | 2069 (1791–2347) | 1504 (1404–1605) | 1171 (1119–1223) | 1750 (1685–1815) | |
| per day | 8.81 (6.39–11.21) | 7.7 (6.7–8.7) | 6.61 (5.78–7.44) | 5.12 (4.38–5.82) | <0.001 | 7.95 (7.58–8.32) |
| Opioid analgesia cost (strong) | ||||||
| per patient | 1723 (1592–1854) | 1863 (1612–2113) | 1239 (1144–1334) | 914 (877–951) | <0.001 | 1495 (1432–1558) |
| per day | 5.7 (4.2–6.5) | 6.1 (5.3–6.9) | 5.3 (4.9–5.7) | 3.6 (3.3–3.9) | <0.001 | 5.7 (5.5–5.9) |
| Healthcare cost excluding analgesia | 5092 (4724–5460) | 6665 (5957–7373) | 6067 (5351–6783) | 4817 (4140–5494) | <0.001 | 5559 (5257–5860) |
| Total healthcare cost | 7126 (6600–7652) | 8734 (7561–9910) | 7571 (6839–8304) | 5988 (5308–6668) | <0.001 | 7309 (6995–7622) |
Values expressed as mean (95% confidence interval) or %.
Online Resource 2 shows the use of healthcare resources during the follow-up period.
Non-healthcare costs (indirect costs)Of the 2832 patients with chronic moderate-to-severe pain due to hip or knee OA, 860 were under the retirement age in Spain (65 years).21 The non-healthcare costs amounted to an average of €12,506 in the period before the index date and €12,227 in the period afterwards. The patients treated with oxycodone had the highest pre- and post-index costs (€15,056 and €14,603, respectively), in comparison with the other groups (Online Resource 3).
DiscussionThis study constitutes a secondary analysis of the OPIOIDS study and is focused on patients with chronic moderate-to-severe pain due to hip or knee OA on treatment with strong opioids. The main strong opioids were fentanyl, tapentadol, oxycodone, and buprenorphine. After 36 months of follow-up, these treatments modestly reduced pain severity, increased cognitive deficit, and slightly increased the percentage of patients with severe-to-total disability, according to the Barthel index.
Patients with chronic moderate-to-severe refractory pain due to hip or knee OA had an average of 3 additional comorbidities, with the most frequent being hypertension, dyslipidemia, and osteoporosis. Our results are in line with previous studies in patients with OA.22–24 Recently, a meta-analysis observed that around 49% of patients with OA had at least 2 comorbidities, mainly hypertension (50%) and dyslipidemia (48%).24 Of note, the prevalence of obesity in this population (24.1%) was higher than in the general Spanish population (17.4%), according to the National Health Survey.25 Calders et al. noted an association between having at least 1 comorbidity and the worsening of pain and/or performance-based physical functioning.22
Our results showed that treatment adherence and MPR did not differ among the treatment groups, and were slightly lower than those reported in patients with hip or knee OA and chronic pain in the OPIOIDS study (37.6% and 18.8%, respectively).2 In line with these assessments, Shcherbakova et al. estimated that 40.4% of patients with chronic pain receiving a buprenorphine-containing pharmacotherapy were adherent after the first year.26 The authors recommended to inform patients about the use of opioids and set up alternative methods to manage pain before prescribing opioids.26
Our results showed that the use of strong opioids for 36 months reduced pain severity by 13.7% (NRS score). This is in agreement with the results in the overall OA patient population with chronic pain, which showed a reduction of 16.9% in pain severity after 1 year of opioid treatment.2 We also reported a 5.3% increase in the prevalence of cognitive deficit, which is higher than that recorded in patients with hip or knee OA and chronic pain in the OPIOIDS study (2.2%).2 In addition, we recorded a 1.6% reduction in the Barthel index, similar to that seen in the OPIOIDS study (−1.1%),2 that was responsible for a slightly increase in the prevalence of severe-to-total disability (1.8%) in a relatively short period of treatment time. These results are in line with the most recent publications that indicate that although opioids may reduce pain while improving functionality in patients with OA, they also may have important adverse events.27,28 Fuggle et al. supported the use of opioids for short time periods after other analgesic options fail, in line with many international and national guidelines.27
OA utilizes a considerable number of healthcare resources, with a relevant economic impact to the healthcare systems and society.29 This impact is particularly high for patients prescribed strong opioids.10 Our study showed that productivity loss accounts for 82% (€12,227) of the total cost associated with the management of patients with hip or knee OA and chronic moderate-to-severe refractory pain, with a small reduction in productivity losses. This means that strong opioid therapy was not able to reduce the existing absenteeism before starting this class of analgesic drugs. Although indirect costs accounted for most of the overall cost, healthcare costs were also responsible for a substantial burden to the healthcare system. This burden was seen not in healthcare costs but rather in higher utilization of healthcare resources such as medical specialist visits, days of hospitalization, emergency room visits, or MNRI tests. Such increased utilization of healthcare resources can be partially explained through switches in patient management from a family physician to pain clinic or pain specialist in rheumatology or orthopedics. These findings were observed in the general findings of the OPIOIDS study,2,10 and have also been reported by others analyzing the utilization of opioid drugs in knee and/or hip OA.7,8 For example, Kern et al. reported larger increases in healthcare resource utilization in US patients taking opioids chronically that never reverted to pre-index date usage.30 Chang et al.31 found that individuals with high-risk prescription opioid use have significantly higher healthcare costs and utilization than their counterparts, especially those with chronic high-dose opioid use.
This study was not without the limitations inherent to retrospective studies, such as underreported diseases, professional/patient variability, the measurements of the main variables, and classification bias. In addition, our results may be influenced by possible inaccuracies in the OA diagnosis coding. Patients with missing/inconsistent data were excluded from the analysis but, due to the low number seen, it is not believed to have had an impact on our results. The causes of opioid treatment discontinuation could not be quantified, and non-pharmacological therapies were not considered. Finally, there is a selection bias regarding the attendance of patients to healthcare centers to get their prescriptions, and the physicians who prescribed the treatment, due to the design of the study.
ConclusionsThe treatment with strong opioids implied a high use of resources and increased healthcare costs in patients with hip or knee OA with chronic moderate-to-severe refractory pain. After 36 months, these treatments showed a modest reduction in pain severity, an increase in cognitive deficit, and a slight increase in number of patients with disability. In addition, treatment with strong opioids did not show a relevant reduction in productivity loss or associated costs. Although further studies in clinical practice are required to confirm our results, new therapeutic approaches to the management of pain may be necessary in these patients.
Authors’ contributionsThe study was conceived by ASM (ansicras@atryshealth.com), JRG (javier.rejas@pfizer.com) and IL (Isabel.lizarraga@pfizer.com) ASM, CTT (carlostornero@gmail.com), FVN (fvargasnegrin@gmail.com), JRG, ASN (arsicras@atryshealth.com) and IL participated and contributed to the design of the study. Data collection and statistical analysis was made by ASM and ASN. Interpretation of data was made by all authors. All authors drafted or revised critically and approved the final version of submitted manuscript.
FundingThe study was funded by Pfizer, SLU.
Conflict of interestsThe authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Antoni Sicras-Mainar and Aram Sicras-Navarro are employees of Atrys Health SA who were paid consultants to Pfizer in connection with this manuscript. Javier Rejas-Gutiérrez and Isabel Lizarraga are employees of Pfizer, SLU. Carlos Tornero-Tornero and Francisco Vargas-Negrín declare that they have no competing interests.
Not applicable.