Understanding the disease activity is fundamental to improve patient prognosis and patients’ quality of life. MiDAS study described disease activity in ankylosing spondylitis (AS) Spanish patients and the proportion of them with controlled disease.
MethodsObservational, cross-sectional, multicenter study carried out under conditions of routine clinical practice. Adult (≥18 years) patients with ≥6 months since AS diagnosis treated ≥3 months prior to inclusion. The primary endpoint was the percentage of patients with low disease activity assessed through BASDAI (primary endpoint) and ASDAS-CRP (secondary endpoint).
Results313 AS patients included: 75.7% male; 78.5% HLA-B*27 positive; mean (SD) baseline age of 50.4 (12.0) years; mean (SD) disease duration of 15.5 (11.6) years; 73.5% were treated with biological disease-modifying antirheumatic drugs (DMARDs), 22.4% with non-biological DMARDs and 53.7% with non-steroidal anti-inflammatory drugs, alone or in combination. Monotherapy with biologics and non-biologics was used by 29.7% and 26.8% of patients, respectively. According to BASDAI, 38.0% were in remission (BASDAI≤2) and 64.5% showed adequate disease control (BASDAI<4). According to ASDAS-CRP, 29.4% achieved remission (ASDAS-CRP<1.3) and 28.1% low disease activity (1.3≤ASDAS-CRP<2.1).
ConclusionsAlmost two thirds of the AS patients recruited had low disease activity, with about one third of them being in remission (BASDAI≤2, ASDAS-CRP<1.3). These results highlight the existing room for improvement in treating AS patients in clinical practice.
Comprender la actividad de la enfermedad es fundamental para mejorar el pronóstico y la calidad de vida de los pacientes. El estudio MiDAS describió la actividad de la enfermedad en pacientes españoles con espondilitis anquilosante (EA) y la proporción de ellos con enfermedad controlada.
MétodosEstudio observacional, transversal, multicéntrico, realizado en condiciones de práctica clínica habitual. Pacientes adultos (≥18años) con ≥6meses desde el diagnóstico de EA tratados ≥3meses antes de la inclusión. La variable principal fue el porcentaje de pacientes en baja actividad, evaluado mediante BASDAI (variable principal) y ASDAS-CRP (variable secundaria).
ResultadosHubo 313 pacientes con EA incluidos: 75,7% varones; 78,5% HLA-B*27 positivos; edad media (DE) basal de 50,4 (12,0) años; duración media (DE) de la enfermedad de 15,5 (11,6) años; el 73,5% fueron tratados con fármacos antirreumáticos modificadores de la enfermedad (FAME) biológicos, el 22,4% con FAME no biológicos y el 53,7% con antiinflamatorios no esteroideos, solos o en combinación. La monoterapia con biológicos y no biológicos fue utilizada por el 29,7 y el 26,8% de los pacientes, respectivamente. Según BASDAI, el 38,0% estaban en remisión (BASDAI≤2) y el 64,5% mostraron un adecuado control de la enfermedad (BASDAI<4). Según ASDAS-CRP, el 29,4% alcanzaron remisión (ASDAS-CRP<1,3) y el 28,1% baja actividad de la enfermedad (1,3≤ASDAS-CRP<2,1).
ConclusionesCasi dos tercios de los pacientes con EA incluidos presentaban baja actividad de la enfermedad, con aproximadamente un tercio de ellos en remisión (BASDAI≤2, ASDAS-CRP<1,3). Estos resultados destacan el margen de mejora existente para tratar pacientes con EA en la práctica clínica.
Ankylosing spondylitis (AS), a common type of axial spondyloarthritis (axSpA), is a chronic inflammatory disease involving mainly the spine and sacroiliac joints, entheses and, less often, peripheral joints, causing inflammation, stiffness and pain and leading to functional impairment and disability.1 About 20–30% of patients are also affected by peripheral arthritis.1,2
AS prevalence has been estimated between 0.1% and 1.4%,3 in our setting between 0.26% and 0.29%,4 quite similar to other European5 and Asian countries.6
Assessment of SpondyloArthritis International Society-European League Against Rheumatism (ASAS-EULAR)7 and Spanish Society of Rheumatology (SER)8 recommendations established as treatment goals for axSpA patients the reduction and/or control of inflammation, pain, stiffness and fatigue, maintenance of spinal flexibility and normal posture, reduction of functional limitations, maintenance of social relationships and work ability and lessening disease complications. To deal with these objectives, ASAS Group defined a core set of measures recommended for patients follow-up.9 Also, ASAS-EULAR recommendations did recommend a treat-to-target approach, but ACR/SAA/SPARTAN did not.10,11 In clinical practice, ASAS-EULAR and SER recommend Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Disease Activity Score preferentially using C-reactive protein (ASDAS-CRP) to assess disease activity in axSpA patients.7,12–14
Biologic disease-modifying antirheumatic drugs (bDMARDs) as tumor necrosis factor inhibitors (TNFi) and interleukin 17 inhibitors have transformed the treatment options especially for those patients with high disease activity. ASAS/EULAR and SER recommend the use of biologics for patients with high disease activity despite conventional treatment, which includes non-steroidal anti-inflammatory drugs (NSAIDs) and non-biologic disease-modifying antirheumatic drugs (nbDMARDs), in case of some concomitant peripheral or extraarticular manifestations.7 However, not all patients have their disease controlled, indicating a need for alternative therapies.7,8
The Atlas 2017 survey15 contributed to the understanding of the reality of people suffering from axSpA and revealed a long diagnostic delay, high disease activity, psychological distress and an important proportion of these patients being undertreated. Also REGISPONSER,16 a Spanish registry of axSpA patients, provided data on the clinical and demographic profile of these patients, including disease activity, in clinical practice. However, data from this type of research is complemented by Real-World Evidence (RWE) studies.
Some studies in AS patients have been carried out regarding different outcomes (disease burden, physical function, quality of life, etc.),17,18 but disease progression remains poorly characterized due to the lack of studies focused on long-term outcomes in clinical practice settings. As a result, the accurate prediction of the AS patients’ outcomes still is an ongoing challenge for clinicians.19 MiDAS emerged from the need to assess the level of disease activity control in AS patients treated in the everyday clinical practice. This data may allow rheumatologists to improve their treatment strategies. The aim of this study was to assess the percentage of AS patients treated in clinical practice who reached low disease activity or remission.
Materials and methodsStudy designMIDAS is a non-interventional, cross-sectional, retrospective, and multicenter study conducted in 36 centers with outpatient rheumatology clinics in Spanish public hospitals between December 10th, 2018 and August 14th, 2019.
Two different cohorts including patients with AS and patients with psoriatic arthritis were studied; here, we present the results of the AS population. The main objective was to evaluate the percentage of AS patients with low disease activity and remission in clinical practice based on the BASDAI and ASDAS-CRP scores, according to present recommendations.10,14
Cross-sectional data were collected during a single routine clinical visit including the primary endpoints as well as patients’ questionnaires and questions about their perception on disease and pain control (secondary endpoints). Retrospective data from medical records and laboratory tests performed prior to patient's inclusion, were also used and were recorded in an electronic Case Report Form specifically designed for MIDAS study.
Eligible patients were ≥18 years old with confirmed diagnosis of AS/r-axSpA for ≥6 months before the inclusion, were classified by the modified New York criteria and ASAS criteria, were treated for ≥3 months and had a record of C-reactive protein (CRP) available in the month prior to the study visit. Patients with severe concomitant diseases that could influence the evaluation of the rheumatic disease (neoplasia, other inflammatory diseases, etc.) were excluded, as well as those participating in any other clinical trial at the time of the inclusion. Patients were required to provide informed consent prior to the study inclusion.
Each center included patients from its databases who met all the selection criteria, in a randomized way. For those centers where random selection was not possible, inclusion was carried out consecutively according to the scheduled visits.
The Spanish version of BASDAI20 was used to assess the percentage of AS patients who presented controlled disease activity. According to this version and following the system adopted by some authors,21 the average punctuation for each of the 6 questions was considered as final score, with a resulting index score (from 0 to 60) which was divided by 6 to obtain a final BASDAI score (from 0 to 10). In MiDAS study, BASDAI<4 was considered as low disease activity and BASDAI≤2 as remission; since SER recommendations considered BASDAI<4 as an acceptable control of the disease, this cutoff was used to define controlled disease.14 For ASDAS-CRP score, the cutoffs for low disease activity (ASDAS-CRP<2.1) and inactive disease (ASDAS-CRP<1.3) were applied.
The study was performed according to the guidelines on observational post-authorization studies for medicinal products for human use specified in Order SAS/3470/2009 of the Spanish Agency of Medicines and Medical Devices and conducted according to Good Clinical Practice (International Conference of Harmonization) guidelines, the Declaration of Helsinki and local regulations, including privacy laws, at the time of the initiation of the study. The study protocol, informed consent forms and information for patients were approved by the Ethical and Clinical Research Committee of the 12 de Octubre Hospital (approval number 18/437).
Statistical analysisNational prevalence for AS was estimated around 0.29%,4 and internal data estimated that 50% of AS patients were on treatment. The proportion of patients with BASDAI<4 was expected in a conservative estimation to be close to 50% to allow the maximum sample size. A minimum of 267 patients was considered necessary to estimate the primary endpoint with a precision of ±6% in its 95% confidence interval; assuming that 15% of patients may not meet the inclusion/exclusion criteria, a predicted number of 315 patients should be recruited to ensure enough power and a good precision.
Continuous variables were described by mean, standard deviation (SD), median, minimum, maximum and, depending on the distribution of the analyzed variable, quartiles. Descriptive analysis was based on evaluable data per parameter, excluding patients with missing values. Data were analyzed with Statistical Analysis System Enterprise Guide 7.15.
ResultsA total of 336 subjects were included in the study, 313 (93.2%) of them evaluable. 23 patients were considered non-evaluable due to not meeting inclusion and/or exclusion criteria and/or incomplete study data.
Baseline characteristicsMean (SD) age of the patients was 50.4 (12.0) years, mainly male (75.7% [237/313]), 39.9% (116/313) were overweight and 75/313 (24.0%) patients were active smokers. Mean (SD) disease duration was 15.5 (11.6) years and mean time (SD) between symptoms’ onset and diagnosis was 5.0 (7.2) years (Table 1).
Baseline demographic and clinical characteristics of the evaluable population.
| AS patients(N=313) | |
|---|---|
| Sociodemographic data | |
| Age (years), mean (SD) | 50.4 (12.0) |
| Sex (male), n (%) | 237 (75.7%) |
| BMI (kg/m2), mean (SD) | 27.0 (4.9) |
| Low weight (BMI<18.5), n (%) | 8 (2.7%) |
| Normal weight (18.5≤BMI<25), n (%) | 100 (34.4%) |
| Overweight (25≤BMI≤30), n (%) | 116 (39.9%) |
| Obesity (BMI>30), n (%) | 67 (23.0%) |
| Missing, n | 22 |
| Smoking habit | |
| Active smoker, n (%) | 75 (24.0%) |
| Packets/year (smokers), mean (SD) | 13.6 (11.2) |
| Former smoker (without smoking>6 months), n (%) | 81 (25.9%) |
| Non-smoker, n (%) | 137 (43.8%) |
| Not available, n | 20 (6.4%) |
| Employment situation | |
| Unemployed, n (%) | 21 (6.7%) |
| Employee (excluding sick leave due to AS), n (%) | 188 (60.3%) |
| On sick leave (due to AS), n (%) | 11 (3.5%) |
| Retired, n (%) | 49 (15.7%) |
| Other (e.g. students, housework, etc.), n (%) | 9 (2.9%) |
| Not available, n (%) | 34 (10.9%) |
| Clinical data | |
| Family history of AS, n (%) | 66 (21.1%) |
| Time of evolution of AS, years, mean (SD) | 15.5 (11.6) |
| Time from onset of AS symptoms, years, mean (SD) | 20.5 (12.7) |
| Time from AS symptoms’ onset to diagnosis, years, mean (SD) | 5.0 (7.2) |
| Family history of psoriasis, n (%) | 43 (13.7%) |
| Time of evolution of psoriasis, years, mean (SD) | 9.1 (3.2) |
| HLA-B*27 | |
| Positive, n (%) | 245 (78.5%) |
| Negative, n (%) | 44 (14.1%) |
| Not available, n (%) | 24 (7.7%) |
| CRP, mg/dl, mean (SD) | 5.1 (8.2) |
| Comorbidities, n (%) | 158 (50.5%) |
| Hypertension, n (%) | 66 (21.1%) |
| Dyslipidemia, n (%) | 61 (19.5%) |
| Diabetes mellitus, n (%) | 25 (8.0%) |
| Cardiovascular disease, n (%) | 18 (5.8%) |
| Osteoporosis, n (%) | 10 (3.2%) |
| Kidney disease, n (%) | 8 (2.6%) |
| Hepatic steatosis, n (%) | 7 (2.2%) |
| Othersa, n (%) | 110 (35.2%) |
At baseline, 158/313 (50.5%) patients had comorbidities, the most frequent being hypertension (21.1% [66/313]), dyslipidemia (19.5% [61/313]) and diabetes mellitus (8.0% [25/313]). Human leukocyte antigen-B*27 (HLA-B*27) was available in 92.3% (289/313) of the patients and was positive in 78.5% (245/313). The most recent mean (SD) CRP value was 5.1 (8.2) mg/dl (Table 1).
Monotherapy with biologicals and non-biologicals was used in 29.7% (93/313) and 26.8% (84/313) of the patients, respectively, while 43.5% (136/313) received a combination of both therapies. Overall, alone or in combination, 73.2% (229/313) of the patients were treated with biologicals and 70.3% (220/313) with non-biologicals (53.7% [168/313] with NSAIDs and 22.4% [70/313] with nbDMARDs) (Table 2). Regarding prescription of bDMARDs, the most frequently used were TNFi (87.8% [201/229]), followed by secukinumab (11.4% [26/229]); adalimumab (31.9% [73/229]) and etanercept (19.2% [45/229]) were the most frequently used among TNFi users. On the other hand, the most prescribed non-biological treatments alone or combination with bDMARDs were NSAIDs (76.4% [168/220]; COX-2 selective inhibitors were the most frequently used NSAIDs: 51.2% [86/168]), followed by DMARDs (31.8% [70/220]).
Treatments used at the initial visit.a
| AS patients(N=313) | |
|---|---|
| Biological treatment, n (%) | 229 (73.2%) |
| Adalimumab, n (%) | 73 (23.3%) |
| Etanercept, n (%) | 45 (14.4%) |
| Golimumab, n (%) | 37 (11.8%) |
| Infliximab, n (%) | 29 (9.3%) |
| Secukinumab, n (%) | 26 (8.3%) |
| Certolizumab pegol, n (%) | 17 (5.4%) |
| Ustekinumab, n (%) | 2 (0.6%) |
| Non-biological treatment, n (%) | 220 (70.3%) |
| NSAIDs, n (%) | 168 (53.7%) |
| COX-2, n (%) | 86 (27.5%) |
| Propionic acid derivatives, n (%) | 35 (11.2%) |
| Acetic acid derivatives and acetamide, n (%) | 27 (8.6%) |
| Oxicam, n (%) | 7 (2.2%) |
| Others, n (%) | 17 (5.4%) |
| DMARDs, n (%) | 70 (22.4%) |
| Methotrexate, n (%) | 30 (9.6%) |
| Sulfasalazine, n (%) | 42 (13.4%) |
| Systemic corticosteroids, n (%) | 15 (4.8%) |
Mean (SD) time elapsed from the start of treatment to the study visit was 65.6 (51.9) months for biological treatments and 75.2 (77.3) months for nbDMARDs, 74.3 (93.4) months for NSAIDs and 64.8 (92.9) months for corticosteroids.
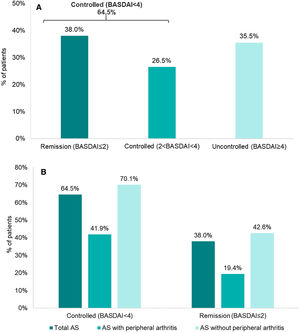
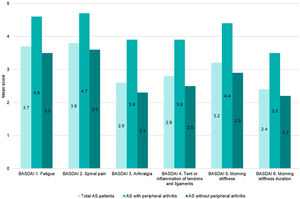
Disease activity controlAccording to BASDAI, 64.5% (202/313) of the patients showed adequate disease control (BASDAI<4), 38% (119/313) were in remission (BASDAI≤2) and 26.5% with controlled disease (2<BASDAI<4) (Fig. 1). Overall, mean (SD) BASDAI score was 3.1 (2.2). The single BASDAI items with highest scores were fatigue and spinal pain, followed by morning stiffness (Fig. 2).
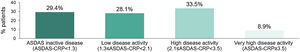
Mean (SD) ASDAS-CRP score was 1.9 (1.1); 29.4% (92/313) of the patients reached inactive disease status (ASDAS-CRP<1.3) and 28.1% (88/313) low disease activity (1.3≤ASDAS-CRP<2.1), while 33.5% (105/313) had high disease activity (2.1≤ASDAS-CRP<3.5) and 8.9% (28/313) very high disease activity (ASDAS≥3.5) (Fig. 3).
When analyzing subgroups according to the presence/absence of peripheral disease, the percentage of disease control according to BASDAI was lower for patients with peripheral involvement (41.9% [26/62]) versus the subgroup without peripheral manifestations (70.1% [176/251]) (Fig. 1).
Both, BASDAI and ASDAS-CRP, showed a higher value in those patients with peripheral arthritis, mean (SD) of 4.2 (2.4) and 2.4 (1.1), respectively. In patients without peripheral manifestations, mean (SD) BASDAI and ASDAS-CRP scores were 2.8 (2.1) and 1.8 (1.0), respectively.
It should be noted that, each of the 6 items evaluated by BASDAI were higher in the subgroup with peripheral disease versus the subgroup without peripheral arthritis (Fig. 2).
DiscussionMiDAS study was designed to assess the disease activity state of AS patients treated in routine clinical practice in Spain. The study shows that 64.5% of the patients achieved low disease activity status (BASDAI<4) and 38.0% were in remission (BASDAI≤2), while 57.5% and 29.4% of the patients had low disease or inactive disease, respectively, according to ASDAS-CRP. This data is essential for the treating rheumatologist to understand the need for treatment optimization and continuous improvement of patient care.
Treatment target for AS patients is to achieve a state of inactive disease or low disease activity.7,10 In clinical practice, disease activity assessment is usually estimated by two scores: BASDAI,12 which contains only subjective clinical elements, and ASDAS-CRP,7,13 which incorporates one objective inflammation measure. In this sense, SER indicated that BASDAI≤2 can be considered as remission, while BASDAI<4 is considered a reasonable control of disease activity.14 Even though BASDAI has been historically widely used to define disease activity in AS patients, ASAS-EULAR consider ASDAS score as the preferred measure since it combines patient-reported outcomes and CRP.7 Also, SER considers ASDAS-CRP as the main index to monitor disease activity, considering acceptable an ASDAS-CRP<2.1, although the therapeutic objective is to achieve an ASDAS-CRP<1.3.14
Different studies have compared and correlated the two outcomes measures used in our study.22,23 The evidence accumulated supports the better discriminatory ability of ASDAS-CRP as a measure of disease activity in AS patients, as well as for selecting patients for TNFi treatment.22,23 In our study, ASDAS-CRP revealed a lower proportion of patients with low disease activity than those determined by BASDAI (57.5% versus 64.5%, respectively), aligned with a study performed in real world setting.24
The percentage of patients with BASDAI<4 (64.5%) is higher than what has been previously reported ranging from 42% to around 50%.25–27 Similarly, the proportion of patients reaching low disease activity based on the ASDAS-CRP score (57.5%) is slightly higher than the reported in previous studies, ranging from 42% to 52.28,29 These results could be explained by a higher introduction of the treat to target strategies in clinical practice or a higher use of bDMARDs in active AS patients, aligned with the results of a recent RWE study which showed a better control of clinical symptoms in patients under TNFi treatment than those treated with NSAIDs.30
The limitations of the MiDAS study include the retrospective, cross-sectional design which does not collect longitudinal data to assess changes over time. Furthermore, since various treatments were not equally distributed within the study population, the effectiveness of the most frequently used would have influenced the results significantly; however, this reflects the reality of the current clinical practice in Spain. Finally, as the patients included in this study were attending outpatient clinics from tertiary reference hospitals, they may represent a population with more comorbidities and higher disease severity; therefore, cautious generalizability to the broader, average AS population, is needed.
ConclusionsThe MiDAS study, by applying widely accepted outcome measures for disease control and remission as BASDAI and ASDAS-CRP, showed that two thirds of the AS patients achieved low disease activity, with one third being in remission. These findings highlight that there is still room for improvement in the management of these patients in the everyday clinical practice in Spanish public hospitals and raise awareness that treatment optimization strategies are needed to improve patient care.
Funding statementThis study was funding by Novartis Farmacéutica, S.A., who has been involved during all the process of the study, including this manuscript.
Conflict of interestEugenio de Miguel reports personal fees from Novartis, during the conduct of the study; grants and personal fees from Novartis, grants and personal fees from Abbvie, grants and personal fees from Pfizer, personal fees from MSD, personal fees from BMS, personal fees from Janssen, grants and personal fees from Roche, personal fees from UCB, personal fees from Lilly, personal fees from Galapagos, outside the submitted work.
Cristina Fernández-Carballido reports personal fees from Novartis, during the conduct of the study; personal fees from Abbvie, personal fees from Celgene, personal fees from Janssen, personal fees from Lilly, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Roche, personal fees from UCB, outside the submitted work.
Jordi Gratacós reports personal fees from Novartis, during the conduct of the study; grants and personal fees from MSD, grants and personal fees from Pfizer, grants and personal fees from Abbvie, grants and personal fees from Janssen, grants and personal fees from Lilly, grants and personal fees from Amgen, outside the submitted work.
José L. Pablos reports personal fees from Pfizer, personal fees from Novartis, personal fees from Roche, personal fees from Abbvie, personal fees from Sanofi, personal fees from Bristol, personal fees from Gilead, personal fees from Galapagos, during the conduct of the study.
Xavier Juanola reports and Personal Fees: Monies paid to you for services rendered, generally honoraria, royalties, or fees for consulting, lectures, speakers bureaus, expert testimony, employment, or other affiliations.
Rafael Ariza has nothing to disclose.
Pau Terradas-Montana, Cristina Sanabra and Carlos Sastré report personal fees from Novartis Farmacéutica S.A, outside the submitted work.
The authors would like to thank all investigators who participated in the MIDAS study (Supplementary material) and IQVIA and Carmen Barrull, Carlos Miñarro and Elena Torres for providing medical editorial assistance with this manuscript.