Systemic lupus erythematosus (SLE) is an autoimmune with variable severity, common in Hispanic and African-American individuals.
ObjectiveTo know the clinical activity and the accumulated damage, as well as the prevalence and incidence, in a dynamic cohort of patients with SLE from the Yucatan Peninsula (1995–2016).
Patients and MethodsA cohort of 200 patients with SLE, medical service beneficiaries of the ISSSTE Regional Hospital of Mérida, Yucatán, was analysed for 22 years. Disease activity and accumulated damage were evaluated using the MEX-SLEDAI scale and the SLICC-ACR-DI, respectively, and its correlation with clinical and demographic variables.
Results185 female and 15 male patients were analysed. Average accumulated damage and activity indices during follow-up were 4.63 and 1.10, respectively. The activity index was significantly lower in females compared to males (4.36 vs 7.43), and the accumulated damage did not present a difference by sex. The manifestations associated with greater activity were the mucocutaneous and articular ones, and the organs with the greatest accumulated damage were the musculoskeletal, neurological and gonadal. A relationship between the indices was found with the evolution time, remissions/reactivations, and persistent activity. Mortality was related to persistent activity due to systemic vascular complications and kidney and liver failure. The annual incidence and prevalence of SLE calculated was 2.86% and 48.43% in Yucatán Peninsula.
ConclusionsThe patients presented persistent activity, with mild to moderate reactivations, and accumulated damage more aggressive in men. The clinical activity decreases and increases the accumulated damage at a longer evolution time, with less kidney disease and greater survival, which suggests a more benign course in the population of the Yucatan Peninsula.
El lupus Eritematoso Sistémico (LES) es una enfermedad autoinmune con severidad variable, frecuente en individuos hispanos y afroamericanos.
ObjetivoConocer la actividad clínica y el daño acumulado, así como la prevalencia e incidencia, en una cohorte dinámica de pacientes con LES de la Península de Yucatán (1995 a 2016).
Pacientes y MétodosSe analizaron 200 pacientes con LES, beneficiarios del servicio médico del Hospital Regional ISSSTE de Mérida, Yucatán, durante 22 años. Se evaluó la actividad de la enfermedad y el daño acumulado mediante la escala MEX-SLEDAI y SLICC-ACR-DI, respectivamente, y su correlación con variables clínicas y demográficas.
ResultadosSe analizaron 185 pacientes femeninos y 15 masculinos. Los índices promedio de actividad y daño acumulado durante el seguimiento fueron de 4.63 y 1.10, respectivamente. El índice de actividad se observó significativamente menor en los femeninos respecto a los masculinos (4.36 vs 7.43), y el daño acumulado no presentó diferencia por sexo. Las manifestaciones asociadas con mayor actividad fueron las mucocutáneas y articulares, y los órganos con mayor daño acumulado el musculoesquelético, neurológico y gonadal. Se encontró relación de los índices con el tiempo de evolución, las remisiones/reactivaciones, y actividad persistente. La mortalidad se relacionó con actividad persistente por complicaciones vasculares sistémicas e insuficiencia renal y hepática. La incidencia y prevalencia anual de LES calculada fue de 2.86% y 48.43% en la Península de Yucatán.
ConclusionesLos pacientes presentaron actividad persistente, con reactivaciones leves a moderadas, y daño acumulado más agresivo en hombres. La actividad clínica disminuye e incrementa el daño acumulado a mayor tiempo de evolución, con menor afección renal y mayor sobrevida, lo que sugiere un curso más benigno en la población de la Península de Yucatán.
Systemic lupus erythematosus (SLE) is a chronic, inflammatory, autoimmune disease of unpredictable course, alternating with remissions and exacerbations, in which multiple organs are affected with variable severity. It predominantly affects women of reproductive age, with a prevalence of approximately 1 in 1000, depending on race and socioeconomic status, 9 times higher incidence in women than in men, more frequent in non-Caucasians (Hispanics and African-Americans) and occupies an important place in the differential diagnosis of patients with multisystem diseases1,2.
The extreme variability of SLE in its clinical expression between individuals, as well as in different ethnic and racial groups, and its traditionally unpredictable course with unpredictable reactivations and remissions, have made it necessary to know the overall disease activity over time, as the severity and duration of the active inflammatory process in a particular affected organ is an important predictor of organ damage and mortality. Disease activity and cumulative damage are important in assessing prognosis3.
The Mexican SLE activity index (MEX-SLEDAI), suitable for assessing activity in SLE patients, is a simplification of the SLEDAI, in which some manifestations such as fatigue are added and headache, visual disturbances and immunological tests such as complement and anti-DNA antibodies are excluded4,5. The cumulative damage index, developed by international clinical collaborators of the American College of Rheumatology (SLICC-ACR-DI), is a validated instrument to measure damage, regardless of cause6,7.
Cross-sectional and retrospective studies have assessed variables that are predictive or associated with harm. The Latin American Group for the Study of Lupus (GLADEL) and the Lupus in Minorities Registry (LUMINA) have reported that disease manifestations and severity may vary in different racial/ethnic populations and socioeconomic status. Latin American, mestizo (mixed Amerindian and European descent) and African-American patients may develop the disease at a younger age, with more severe clinical manifestations, higher activity, cumulative damage and higher activity-related mortality and infections8,9.
Ethnicity plays an important role in the development of SLE, and people of Amerindian origin are more susceptible to developing the disease. In Mexico, women with SLE appear to have more severe disease, a lower age of onset than their European counterparts, as well as a higher frequency of disease activity flares. Significant ethnic variability has been reported among Mexican subpopulations, resulting from the combination of Amerindian, European and, to a lesser extent, African parental groups10,11. The Yucatan subpopulation has a distinctive Amerindian (Mayan) contribution, which tends to decrease towards the north of our country, and in the same sense the European increases proportionally, and they represent an ethnic group geographically distant from other Mexican Amerindian groups12,13. On the other hand, studies conducted in women with SLE from the Mayan subpopulation in Yucatan have shown the involvement of immune mechanisms and genetic factors associated with the development of the disease14–18.
Our objective was to determine the clinical evolution of activity and accumulated organ damage over the years in a dynamic cohort of patients with SLE in the Yucatan peninsula, as well as the prevalence and incidence of the disease.
Material and methodsPatient selectionDescriptive study of a dynamic cohort of 200 entitled patients, who met 4 or more criteria for the diagnosis of SLE and regularly attended the Rheumatology Service of the Regional Hospital of the Institute of Security and Social Services for State Workers (ISSSTE) in Merida, Yucatan, Mexico19. They were evaluated by physical examination and laboratory studies every 3 months, during the period 1995−2016. All patients signed a letter of informed consent to participate in the clinical follow-up, in accordance with the principles of the Declaration of Helsinki20. They were considered mestizo because they were born in the Yucatan peninsula and had ancestors of Amerindian origin, mostly from the Mayan ethnic/racial group.
Estimation of disease activity and cumulative damage indexDisease activity was assessed by applying the MEX-SLEDAI, considering 3 grades: inactive, those patients who remained with a score of 0 for at least one year; reactivations and remissions characterised by periods of reactivation (points > 0) and remissions (points = 0); and persistent activity those who presented a score >0 for one year or more21. Reactivations were subclassified as mild (2–5 points), moderate (6–9 points), severe (10–13 points) and very severe (14 points or more) 22. To assess the cumulative damage index, the SLICC-ACR-DI was applied taking into account the organic damage that occurred after the diagnosis of the disease. Two degrees of damage were considered: stable with an index equal to 0 and increased with a value of ≥1.
Sociodemographic factorsFactors such as gender, age of onset, time of evolution and educational level were included. Educational level was categorised as low (completed studies of 9 years or less; primary and secondary), medium (completed studies of 10–12 years; baccalaureate) and high (completed studies of 13 years or more; bachelor's, master's degree)23.
Therapeutic factorsActivity and cumulative damage rates were related to the drugs used in treatment, which included: prednisone doses/day ≤5 mg, 5–30 mg and ≥30 mg, hydroxychloroquine, azathioprine, methotrexate, mycophenolic acid, cyclophosphamide and methylprednisolone pulses, and biological therapy with anti-CD20 (rituximab).
Prevalence and incidenceThe prevalence and incidence of SLE were estimated for the period January 1999 to December 2016, with reference to the number of beneficiaries receiving health services at the ISSSTE Regional Hospital, Mérida, Yucatán, provided by the statistics department and the institute’s yearbook, including patients from the states of Yucatán, Campeche and Quintana Roo24.
Statistical analysisStatistical analysis was carried out using SPSS® v.22 and S-PLUS® v.6.2 using measures of central tendency, dispersion and percentages, and comparison of averages by means of Student’s t-test and/or ANOVA, in order to establish possible differences between the average values of activity and cumulative damage. The indices were related to the variables sex, age at onset, school level, time of evolution and follow-up of less than and more than 10 years, relapses, persistent activity, remissions/reactivations, the subgroups of mild, moderate and severe activity, as well as the treatment received and deaths. The survival rate of the cohort of patients was evaluated taking into account the time of disease progression.
ResultsFollow-up began in January 1995 and concluded in December 2016. The dynamic cohort consisted of 185 female and 15 male patients from the 3 states that make up the Yucatan peninsula, most of them from the state of Yucatan, followed by Campeche and Quintana Roo. The high school level was most frequently associated with teaching, and the medium and low levels with housework. During follow-up, there were 19 deaths and 25 paediatric patients with an age of onset between 10–16 years (Table 1).
Characteristics of patients with SLE.
| N = 200 | |
|---|---|
| Variables | |
| Female sex | 185 (92.5%) |
| Age (years). Mean ± SD | 44.9 ± 13.2 |
| Age at onset (years). Mean ± SD | 31.2 ± 12.0 |
| Time of evolution, Mean ± SD | 13.8 ± 8.0 |
| >10 years | 113 (56.5%) |
| Time of follow-up (years). Mean ± SD | 12.2 ± 7.0) |
| >10 years | 102 (51%) |
| Relapses, Mean ± SD | 4.2 ± 3.4 |
| Locality | |
| Yucatán | 123 (61.5%) |
| Campeche | 41(20.5%) |
| Quintana Roo | 36 (18.0%) |
| Level of education | |
| Low level | 12 (6.0%) |
| Intermediate level | 83 (41.5%) |
| High level | 105 (52.5%) |
| Paediatric SLE | |
| Male | 4 (2%) |
| Female | 21 (10.5%) |
| Deceased | 19 (9.5%) |
SD: Standard Deviation.
Based on data provided by the yearbook of the Statistics Department of the ISSSTE Merida Regional Hospital, considering an average annual population corrected for ages 10–69 years of 250,788 and 105,582 beneficiaries for the peninsula and the State of Yucatan, respectively, an average prevalence of 48.43 (95% CI: 46.26–50.60) and 69.56 (95% CI: 64.20–74.93) per 100,000 beneficiaries was estimated for the period from January 1999 to December 2016. The average estimated incidence for the same period was 2.86 (95% CI: 2.18–3.54) and 4.31 (95% CI: 3.27–5.36) per 100,000 beneficiaries for the Peninsula and the state of Yucatan, respectively.
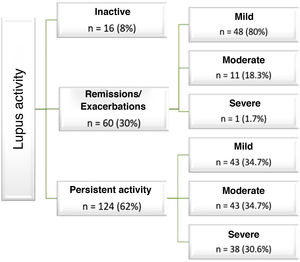
In relation to the disease, 62% of patients had mild, moderate and severe persistent activity. In those who presented exacerbations, these were mostly mild (Fig. 1).
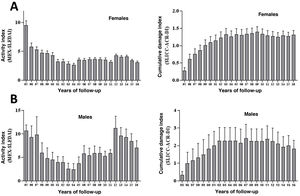
In the female patients we observed a decrease in activity during follow-up, with a tendency towards persistent, mild and moderate activity, and progression of accumulated damage that seems to stabilise from the ninth year of follow-up (Fig. 2A). In men, reactivations were frequent and severe, with an increase in cumulative damage (Fig. 2B).
The mean MEX-SLEDAI in all patients during follow-up was 4.63 ± 2.85, and the clinical manifestations most frequently related to activity were mucocutaneous, immunological and joint manifestations, followed by fatigue/fever, haematological, renal, neurological, vasculitis, serositis and myositis. Regarding immunological markers, 97.5% presented ANA and anti-DNA, at titres greater than 1:80, with a homogeneous diffuse and mottled pattern, and 16.4% had antiphospholipid, anticardiolipin and lupus anticoagulant antibodies. With regard to SLICC-ACR-DI 66% of patients had values ≥1, with an overall mean of 1.10 ± 1.29, and the organs with the most frequent damage were musculoskeletal, neurological and gonadal failure, followed by renal, cutaneous, ocular, cardiovascular, diabetes mellitus, pulmonary, gastrointestinal and neoplasms. Hyperlipidaemia and antiphospholipid syndrome, followed by Rhupus, were the most frequent manifestations associated with SLE (Table 2).
Clinical manifestations and organs related to activity (MEX-SLEDAI) and cumulative damage (SLICC-ACR-DI), respectively, in the cohort of SLE patients.
| N (%) | |
|---|---|
| Clinical manifestations related to the activity | |
| Mucocutaneous | 196 (98) |
| Immunological | 195 (97.5) |
| Arthritis | 168 (84) |
| Fatigue/Fever | 152 (76) |
| Haematological | 86 (43) |
| Renal | 85 (42.5) |
| Neurological | 52 (26) |
| Vasculitis | 46 (23) |
| Serositis | 43 (21.5) |
| Myositis | 16 (8) |
| Cumulative damage index | |
| Incremented (values ≥ 1) | 132 (66) |
| Organs linked with cumulative damage | |
| Musculoskeletal: | 57 (28.5) |
| Muscular atrophy | 23 (11.5) |
| Deforming arthritis | 18 (6.5) |
| Osteoporosis | 12 (6) |
| Avascular necrosis | 4 (2) |
| Premature gonadal failure | 49 (24.5) |
| Renal: | 43 (21.5) |
| glomerular filtering <50% | 13 (6.5) |
| Proteinuria ≥3.5 g/24 h | 25 (12.5) |
| Chronic kidney failure | 15 (7.5) |
| Neurological: | 58 (29) |
| Cognitive damage | 20 (10) |
| Convulsions | 18 (9) |
| Cerebral vascular event | 13 (6.5) |
| Peripheral neuropathy | 6 (3) |
| Transverse myelitis | 1 (0.5) |
| Ocular: | 23 (11.5) |
| Cataract | 18 (9) |
| Retinopathy | 5 (2.5) |
| Skin | 29 (14.5) |
| Scarring alopecia | 7 (3.5) |
| Skin ulcers | 22 (11) |
| Cardiovascular: | 20 (10) |
| Ischaemic heart disease | 5 (2.5) |
| Pericarditis | 12 (6) |
| Valvular disease | 3 (1.5) |
| Peripheral vascular: | 19 (9.5) |
| Minor tissue loss | 5 (2.5) |
| Venous thrombosis | 14 (7) |
| Diabetes mellitus | 15 (7.5) |
| Pulmonary: | 9 (4.5) |
| Pulmonary fibrosis | 2 (1) |
| Collapsed lung | 1 (.5) |
| Pleural fibrosis | 5 (2.5) |
| Pulmonary arterial hypertension | 1 (.5) |
| Gastrointestinal: | 5 (2.5) |
| Splenectomy | 2 (1) |
| Cholecistectomy | 3 (1.5) |
| Neoplasias | 5 (2.5) |
| Other manifestations related to SLE | |
| Hyperlipidaemia | 35 (17.4) |
| Antiphospholipid syndrome | 33 (16.4) |
| Rhupus | 16 (8) |
| Discoid lupus e | 7 (3.4) |
| Sjögren’s syndrome | 3 (1.4) |
The means of the MEX-SLEDAI index were compared with the different variables (Table 3), and we observed that men had higher activity than women. Patients with evolution and follow-up times of less than 10 years had significantly higher activity. Patients with renal, neurological, vasculitis, fatigue/fever and myositis activity were significantly associated with higher disease activity, as were those with more than 3 reactivations during follow-up. No differences were observed in the means of the activity index in relation to school level and age at disease onset. On the other hand, patients with higher mean MEX-SLEDAI received higher doses of prednisone, cyclophosphamide pulses, methylprednisolone, immunomodulators, biologic therapy and were significantly associated with deaths.
Analysis of mean activity index values (MEX-SLEDAI) with various variables of the disease in the total group of patients (n = 200).
| Variables | Mean MEX-SLEDAI | p | |
|---|---|---|---|
| Sex (F: female. M: male) | F = 4.3 | M = 7.5 | .009 |
| Time of SLE onset | Teenage = 5.3 | Adult = 4.4 | .13 |
| Level of education | low = 4.3. intermediate = 4.9. high = 4.3 | .41a | |
| Time of evolution | ≤10 years = 5.7 | >10 years = 3.7 | <.0001 |
| Time of follow-up | ≤10 years = 5.4 | >10 years = 3.7 | <.0001 |
| Relapses | ≤3 = 3.9 | >3 = 5.3 | .001 |
| Clinical manifestations related to the activity | Yes | No | |
|---|---|---|---|
| Mucocutaneous | 4.5 | 5.3 | .61 |
| Immunological | 4.5 | 3.9 | .63 |
| Arthritis | 4.6 | 4.2 | .50 |
| Fatigue/Fever | 4.8 | 3.7 | .02 |
| Haematological | 5.0 | 4.2 | .08 |
| Renal | 5.9 | 3.5 | <.0001 |
| Neurological | 6.3 | 3.9 | <.0001 |
| Vasculitis | 6.0 | 4.1 | <.0001 |
| Serositis | 5.2 | 4.4 | .10 |
| Myositis | 8.0 | 4.2 | <.0001 |
| Treatment | |||
| Prednisone mg/day | ≤5 = 2.5◊. 5−30 = 4.7b ≥30 = 7.8b | <.0001a | |
| Azatioprine | 4.6 | 3.1 | .048 |
| Hydroxichloroquine | 4.6 | 4.3 | .66 |
| Cyclophosphamide pulses | 5.3 | 2.9 | <.0001 |
| Methylprednisolone pulses | 5.4 | 3.1 | <.0001 |
| Mycophenoloic acid | 5.5 | 3.8 | <.0001 |
| Methotrexate | 4.6 | 4.5 | .89 |
| Rituximab | 6.2 | 4.3 | .005 |
| Deceased | 7.7 | 4.2 | .001 |
p: Student’s t-test.
When analysing the average SLICC-ACR-DI values with the same variables (Table 4), it can be seen that men show higher damage indices than women, but not significantly, and those with evolution and follow-up of more than 10 years and with more than 3 relapses showed greater organ damage. The most affected organs were musculoskeletal, renal, gonadal, neurological, ocular and cardiac, among others; all with significant differences with respect to those who did not present damage to said organ. The SLICC-ACR-DI averages were significantly higher in those who died and in those who received higher doses of prednisone, immunomodulators, cyclophosphamide pulses, methylprednisolone pulses and biological therapy, compared to those with less activity and accumulated damage.
Analysis of the average values of the cumulative damage index (SLICC-ACR-DI) with various variables of the disease in the total group of patients (n = 200).
| Variables | Mean SLICC-ACR-DI | p | |
|---|---|---|---|
| Sex (F = female, M = male) | F = 1.1 | M = 1.5 | .21 |
| Time of SLE onset | teenage = 1.0 | Adult = 1.1 | .75 |
| Level of education | Low = 1.8b. intermediate = 1.2, high = .93b | .05a | |
| Time of evolution | ≤10 years = .78 | >10 years = 1.3 | .001 |
| Time of follow-up | ≤10 years = .72 | >10 years = 1.48 | <.0001 |
| Relapses | ≤3 = .59 | >3 = 1.72 | <.0001 |
| Organs with cumulative damage | Yes | No | |
|---|---|---|---|
| Musculoskeletal | 1.9 | .78 | <.0001 |
| Premature gonal failure | 2.2 | .75 | <.0001 |
| Renal | 2.1 | .86 | <.0001 |
| Neurological | 2.3 | .73 | <.0001 |
| Ocular | 2.4 | .94 | .001 |
| Skin | 2.6 | .90 | <.0001 |
| Cardiac | 2.9 | .91 | <.0001 |
| Vascular | 2.4 | .98 | .003 |
| Diabetes mellitus | 1.7 | 1.10 | .072 |
| Pulmonary | 2.5 | 1.00 | .001 |
| Gastrointestinal | 2.7 | 1.10 | .006 |
| Neoplasias | 2.7 | 1.10 | .004 |
| Treatment | |||
| Prednisone mg/day | ≤5 = .39b, 5−30 = 1.28b, ≥30 = 2.0b | <.0001a | |
| Azatioprine | 1.1 | .67 | .17 |
| Hydroxichloroquin | 1.2 | .62 | .005 |
| Cyclophosphamide pulses | 1.4 | .47 | <.0001 |
| Methylprednisolone pulses | 1.5 | .49 | <.0001 |
| Mycophenoloic acid | 1.2 | 1.0 | .24 |
| Methotrexate | 1.3 | 1.1 | .41 |
| Rituximab | 1.9 | 1.0 | .02 |
| Deceased | 2.8 | .93 | <.0001 |
p: Student’s t test.
During the follow-up period, the 19 deaths were mainly due to systemic vascular complications, sepsis, and renal and hepatic failure, and involved severe persistent activity. The mean MEX-SLEDAI was significantly higher in those who died than in those who remained alive (7.81 ± 3.82 vs. 4.25 ± 2.48, p = .00079), as was the mean SLICC-ACR-DI index (2.77 ± 1.55 vs. .93 ± 1.13, p = .00006) (Table 5).
Clinical characteristics of those who died during the follow-up of the 200-patient cohort.
| Variables | N (%) |
|---|---|
| Deceased | 19 (9.50) |
| Systemic vasculitis/gastrointestinal sepsis | 7 (36.84) |
| Chronic renal and hepatic failure | 4 (21.05) |
| Melanoma and retroocular tumour | 2 (10.52) |
| Pulmonary thrombosis | 2 (10.52) |
| Central nervous system vasculitis | 2 (10.52) |
| Pneumonitis/respiratory failure | 1 (5.26) |
| Pericarditis | 1 (5.26) |
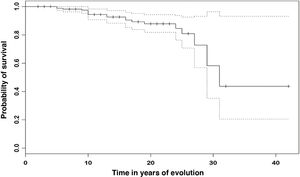
The cumulative individual survival probability over the time of evolution was calculated (Fig. 3), and shows a survival of 98.9% (95% CI 97.3–100 %) at 5 years, 94.4% (95% CI 90.7–98.3 %) at 10 years and 87.8% (95% CI 81.8–94.3 %) at 20 years. At the end of follow-up survival was 43.7% (95% CI 20.5–93.1 %) in those patients with 30 years of evolution.
DiscussionThis is the first study on the follow-up of clinical activity and cumulative damage in a dynamic cohort of entitled patients with a diagnosis of SLE, who regularly attended the rheumatology service of a tertiary level public hospital that receives patients from the 3 states of the Yucatan peninsula in Mexico: Yucatan, Campeche and Quintana Roo. Patients from this region are a little-studied mestizo ethnic/racial group of Mayan Amerindian descent, sharing sociodemographic, cultural, geographic and racial characteristics, different from others in Latin America11,12.
Disease incidence and prevalence have been reported to vary between populations in different geographic areas and racial/ethnic groups. In the USA, during the period 2002–2004, Lim et al. studied 1446 cases of SLE from 2 communities in Georgia (Fulton and DeKalb), and estimated an age-adjusted incidence and prevalence of 5.6/100,000 and 74.4/100,000, respectively, much higher in women of colour than in men25. Somers et al. studied 2139 cases from a sociodemographically diverse population in southeast Michigan, and reported a similar incidence and prevalence of 5.5/100,000 and 72.8/100,000, respectively, with women of colour being more affected26. On the other hand, in the period from 2007 to 2009, Dall'Era et al. analysed 1257 cases from San Francisco, including different ethnic groups such as Caucasians, Asians, African-Americans and Hispanics, and reported an incidence and prevalence in Hispanics of 5.6 and 110.5/100,000 inhabitants, respectively27. In the same period Izmirly et al. studied 1078 cases from New York and observed an incidence and prevalence in Hispanics of 4.1 and 84.6/100,000 respectively28. In Mexico, Peláez-Ballestas et al., studying non-traumatic musculoskeletal pain during the last 7 days in 5 regions of Mexico and the southeast of the country, including Yucatán, by COPCORD methodology report a national SLE prevalence of .06% and in Yucatán of .07% (95% CI: .01–.02)29. In our study, considering the number of patients analysed in the 18-year period (1999–2016), the estimated average annual incidence and prevalence of the disease in a tertiary public health institution in the Yucatan peninsula is lower compared to those mentioned above, which were conducted multicentrically and in an open population. However, the incidence and prevalence in the State of Yucatan tend to be similar, and seem to correlate with the prevalence reported by Pelaez-Ballestas et al.
In relation to disease, despite the sample size and long follow-up period, our data show a higher frequency in female patients (92.5%) compared to male patients (7.5%), and correlate with those reported in the GLADEL (89% vs. 11%) and LUMINA (93% vs. 7%) studies. The age of onset in our patients is associated with the documented reproductive stage and correlates with that reported in these same studies8,9.
During the follow-up of the clinical course, we observed that most of our patients had persistent activity, followed by reactivations/remissions and clinical remission. This clinical behaviour is similar to that reported by Barr et al. who found 58% persistent activity, 26% reactivations/remissions and 16% remissions with global medical assessment, and 40% chronic activity, 35% reactivations/remissions and 25% remissions with MEX-SLEDAI21. On the other hand, in our cohort we observed a delay in diagnosis, with a mean of 6 months from symptom onset, and the most frequent clinical course was persistent activity and mild to moderate reactivations, similar to that reported by Nikpour et al.30 The mean MEX-SLEDAI value in our cohort was 4.63 ± 2.85, lower than that of the GLADEL study of 8.6 ± 4.9, probably related to the fact that in our cohort of patients all had social security medical service and continuous follow-up every 3 months in the rheumatology area. During follow-up, male patients had significantly higher activity rates than female patients, comparable to the study by Molina et al. in male Latin American patients with more severe disease31.
In relation to clinical manifestations related to disease activity, we found a higher frequency of mucocutaneous and fatigue/fever compared to the GLADEL study (90.7% and 52.9%). However, we observed lower frequency of musculoskeletal, renal and haematological manifestations with those reported by LUMINA (93%, 62%, 90%) and GLADEL (92.5%, 58.3%, 74.3%), and neurological and pleuritis was similar to that of GLADEL mestizos (27.7% and 20.9%)8,9.
With regard to SLICC-ACR-DI, the mean value was 1.1070 ± 1.29, higher than that reported by GLADEL (.59 ± 1), probably due to the longer evolution and follow-up time of our cohort. On the other hand, the use of oral and intravenous steroiSD, antimalarials (hydroxychloroquine) and immunosuppressants (azathioprine and cyclophosphamide) in our cohort of patients were slightly higher than in the GLADEL study, which is related to the increase in organ damage and duration of follow-up. However, we observed a 5-year survival rate of 98.9%, higher than the GLADEL study of 95% at 4 years. Musculoskeletal organ damage and gonadal failure did not influence survival, while patients with higher activity and renal, neurological and cardiopulmonary damage received higher doses of steroiSD, immunosuppressants and biologic therapy, which is related to better control of activity and relapse and increased organ damage. 9.5% of deaths were associated with persistent high activity with cumulative organ damage related to systemic vasculitis, gastrointestinal sepsis, chronic renal and liver failure, and to a lesser extent neoplasms.
ConclusionsThe course and clinical presentation during follow-up in the cohort of SLE patients from the Yucatan peninsula appears to be similar to that described in other Latin American populations. However, during follow-up we observed a predominance of persistent activity with mild to moderate reactivations, and more aggressive behaviour in men than in women; with longer evolution and follow-up time, we observed a decrease in clinical activity and an increase in cumulative damage, probably conditioned by treatment. The average MEX-SLEDAI in our study was lower than reported in other cohorts, while the average SLICC-ACR-DI increased during follow-up. The higher observed survival and lower renal involvement suggests a more benign course of the disease in the population of the Yucatan Peninsula, Mexico.
Conflict of interestsAll the authors have no conflict of interests to declare related to this research.
Please cite this article as: López-Villanueva RF, Valencia-Pacheco G, Zapata-Vázquez R, López-Suárez R, Castro-Sansores C. Seguimiento de la actividad clínica y del daño orgánico acumulado en una cohorte de pacientes con lupus eritematoso sistémico de la península de Yucatán, México (1995–2016). Reumatol Clin. 2023;19:106–113.