To evaluate the duration of etanercept (ETN) treatment and motives for discontinuation in our local cohort of patients with rheumatic pathology and compare them to the group with other biological treatments.
Patients and methodsProspective observational cohort study. Disease diagnosis, start and end date and motive for discontinuation were recorded. Survival estimation was explored using Kaplan–Meier analysis with remaining patients censored at 1-year, 2-years and 5-years follow-up.
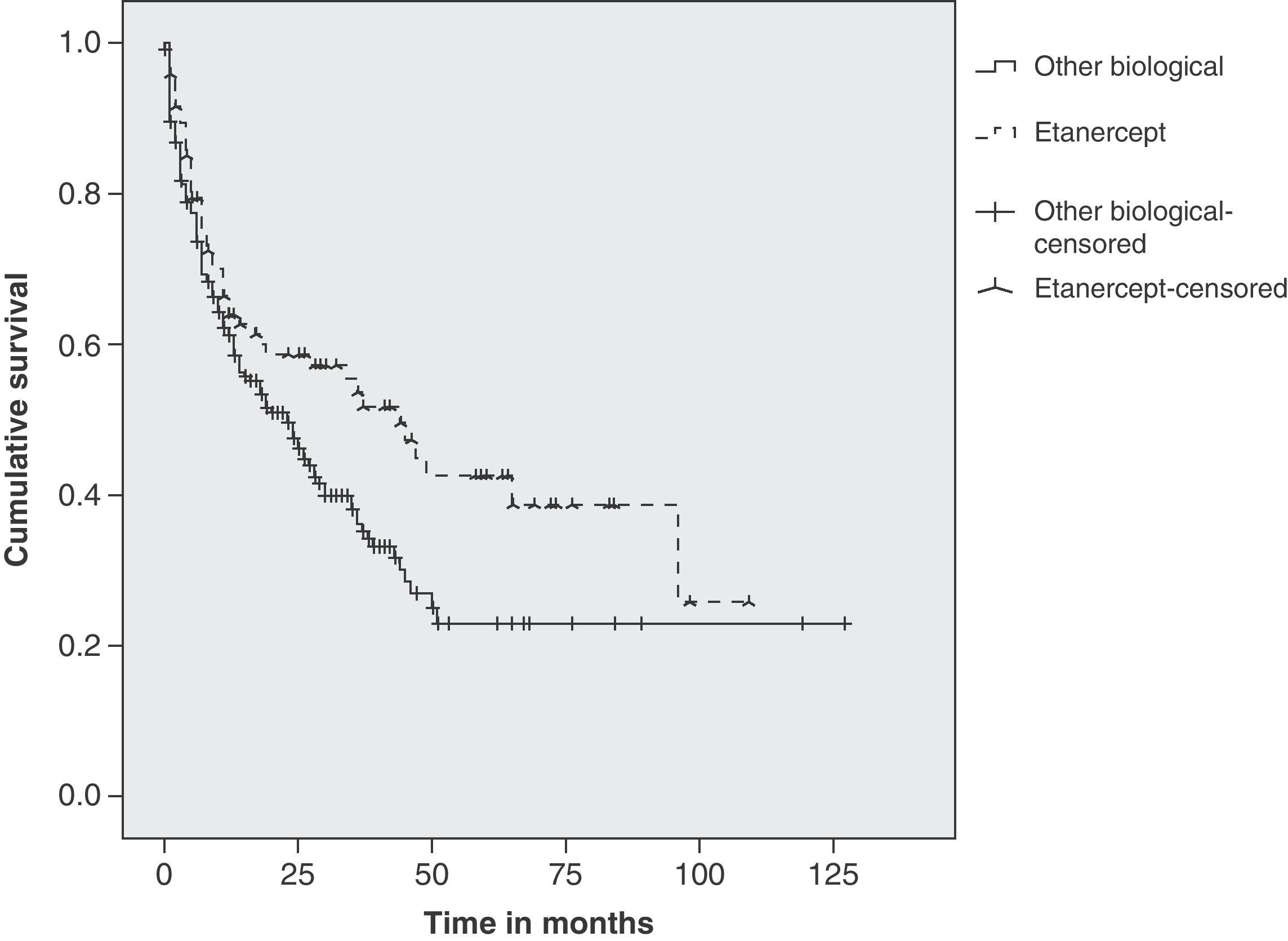
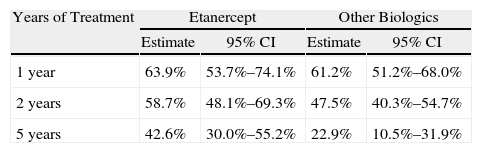
ResultsNinety-two (45%) out of 205 patients started ETN treatment. Disease diagnoses recorded were: 48% rheumatoid arthritis, 33% ankylosing spondylitis, 11% psoriatic arthritis, 8% others (juvenile idiopathic arthritis, inflammatory bowel disease related spondylitis, SAPHO syndrome). 52% of patients are still on the drug. The motives for discontinuation were: inefficacy (65%), adverse events (33%) and lack of compliance (2%). Two patients discontinued ETN due to prolonged disease control. Adverse events were: infection (4 patients), post-injection skin reaction (3), uveitis (3), neoplasia (2) and others (3). Using a Kaplan–Meier analysis, at 1-year 64% (CI95% 54–74) of patients with ETN treatment had not experienced treatment failure, at 2-years, 59% (48–69) and at 5-years, 43% (30–52). With the rest of biologicals estimated survival was 61% (51–68), 47.5% (40–55), and 23% (10.5–32), respectively. Statistical analysis revealed significant differences (log-rank: P=.024; Breslow: P=.068; Tarone-Ware: P=.040).
ConclusionsIn our cohort of patients treated with ETN the estimated survival was better than patients treated with other biological drugs at 1-year, 2-years and 5-years.
Evaluar la supervivencia del tratamiento con etanercept (ETN) y las causas de discontinuación en una cohorte local de pacientes en tratamiento biológico (TB). Comparar con la supervivencia general del resto de TB.
Pacientes y métodosEstudio observacional prospectivo de cohortes. Se han analizado los datos de diagnóstico, fecha de inicio y fin de tratamiento, así como la causa de interrupción de nuestro registro de TB. Mediante el método de Kaplan–Meier se ha estimado la supervivencia de ETN al año, a los 2 años y a los 5 años.
ResultadosDe un total de 205 pacientes que recibieron TB, 92 (45%) iniciaron tratamiento con ETN. En el 48% el diagnóstico fue artritis reumatoide, 33% espondilitis anquilosante, 11% artritis psoriásica y 8% otros diagnósticos (artritis idiopática juvenil, espondiloartritis asociada a enfermedad inflamatoria intestinal y síndrome SAPHO). Continúan con ETN 48 pacientes (52%). Las causas de discontinuación fueron: ineficacia (65%), acontecimiento adverso (33%), pérdida de seguimiento (2%). En 2 pacientes el tratamiento se retiró por remisión clínica. Los acontecimientos adversos fueron: infección (4 pacientes), reacción cutánea post-inyección (3), uveítis (3), neoplasia (2) y otros (3). La supervivencia estimada de ETN al año de tratamiento fue del 64% (IC del 95%, 54-74), a los dos años del 59% (48-69) y a los 5 años del 43% (30-52), y la del resto de TB fue del 61% (51-68), el 47,5% (40-55) y el 23% (10,5-32), respectivamente. Los tests estadísticos revelaron diferencias significativas (log-rank: p=0,024; Breslow: p=0,068; Tarone-Ware: p=0,040).
ConclusionesEn nuestra cohorte de pacientes la supervivencia estimada de ETN en el primero, segundo y quinto de año de tratamiento es superior a la obtenida con el resto de TB, siendo la diferencia significativa a los 5 años.
Biological therapy (TB) was launched by inhibitors of tumor necrosis factor alpha (TNF-α), infliximab (INF), adalimumab (ADA) and etanercept (ETN), and has radically changed the treatment of certain rheumatic diseases such as rheumatoid arthritis (RA), ankylosing spondylitis (AS) or psoriatic arthritis (PsA). The effectiveness of these anti-TNF drugs has been demonstrated in numerous clinical trials, but there are no comparative clinical trials and meta-analysis studies that demonstrate superiority between them.1,2
Recent studies based on national registries of patients with RA and biological therapy have reported lower rates of discontinuation with ETN than with ADA and INF.3–6 Patients with PsA have reported higher compliance rates for ETN and ADA than with INF,7 a fact that has not been confirmed in later studies.8
The aim of our study is to evaluate the duration of treatment with etanercept (ETN) and the causes of its discontinuation in a cohort of patients with biological treatment in an actual clinical practice situation and overall compliance compared with the rest of biological drugs.
MethodsWe performed a prospective, observational cohort study using data from a registry of patients with biological therapy of the section of rheumatology at the Hospital Marina Baixa. This registry began in 2001 to assess the safety and long-term management of these drugs in actual clinical practice and compare it with records from other populations. It includes obtaining informed consent prior to access to medical records of any patient who has received at least one dose of any biological drug prescribed by the section of rheumatology. This was an observational study in a real clinical practice setting, without interference in the selection, initiation or discontinuation of biological treatment.
Patient data were collected by the rheumatologists of the unit in a computer spreadsheet (Microsoft Excel 2003® TM), access to which was restricted in order to comply with the Data Protection Act currently in force.
The study protocol was approved by the local ethics committees and followed the guidelines of the Declaration of Helsinki.
Study VariablesThese included: epidemiological characteristics (age, gender), primary diagnosis, year of diagnosis and result of Mantoux and booster tests. Rheumatoid factor (RF; U/ml) and citrullinated peptide antibodies (anti-CCP) using a second-generation ELISA technique were determined in patients with RA, JIA and PsA. Patients with spondyloarthropathy were analyzed for the presence of histocompatibility antigen HLA-B27.
For each biological agent used we collected the type of drug and start and end dates (and thus the time of treatment). The causes of discontinuation were classified as: inefficiency, complication, clinical remission and loss to follow up.
The different treatments were followed prospectively for each patient, since the start of therapy. In those patients who had previously initiated treatment, for example in another facility, data were included retrospectively.
Statistical AnalysisWe used Kaplan–Meier estimated survival function curves plotted for treatments with ETN and for other biological as a whole. To check for differences between the two groups we used a log-rank, Breslow, and Tarone-Ware tests using the statistical software package SPSS 18®.
ResultsSince 2001, a total of 205 patients started biologic therapy, 92 (45%) patients received ETN (51% as the first biologic drug, 42% as the second drug, 7% as a third drug, and 2% as the fourth drug).
The diagnoses of patients who received ETN were: RA 48%, SA 33%, PsA 11%, and other diagnosis 8% (JIA, spondylitis associated with inflammatory bowel disease and SAPHO syndrome). Currently 48 patients are continuing treatment with ETN (52%).
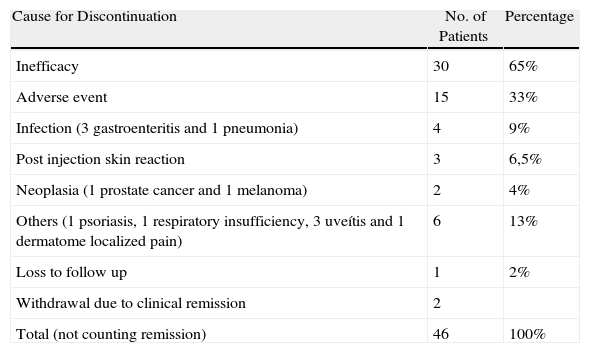
Causes of DiscontinuationThe causes of discontinuation of ETN were as follows (Table 1) ineffectiveness in 30 patients (65%), adverse event in 15 patients (33%) and loss to follow up in 1 case (2%). In 2 patients the treatment was withdrawn after prolonged clinical remission.
Causes for ETN Discontinuation.
| Cause for Discontinuation | No. of Patients | Percentage |
| Inefficacy | 30 | 65% |
| Adverse event | 15 | 33% |
| Infection (3 gastroenteritis and 1 pneumonia) | 4 | 9% |
| Post injection skin reaction | 3 | 6,5% |
| Neoplasia (1 prostate cancer and 1 melanoma) | 2 | 4% |
| Others (1 psoriasis, 1 respiratory insufficiency, 3 uveítis and 1 dermatome localized pain) | 6 | 13% |
| Loss to follow up | 1 | 2% |
| Withdrawal due to clinical remission | 2 | |
| Total (not counting remission) | 46 | 100% |
Adverse events reported were: infection in 4 patients, post-injection skin reaction in 3 patients, malignancy in 2 patients and other causes in 6 patients.
Of the total group of patients with biologic therapy, 7 (3%) interrupted their treatment due to prolonged clinical remission as evaluated by the rheumatologist. Of these, 4 patients resumed the same treatment due to worsening and were counted as “continuation” for the calculation of survival.
In 6 patients treatment was discontinued due to other causes (pregnancy, headache with numbness, nasopharyngeal granulomatosis, myocardial infarction, unstable angina and unknown causes) and was later restarted. These cases were also counted as “continuation” of treatment. Discontinuations due to infection were recorded as ‘discontinued’, although treatment was restarted a few months later.
Two patients dropped out for personal reasons and subsequently restarted the same biologic drug and were counted as “discontinuation” and then as starting new treatment.
Duration of Treatment and Estimated SurvivalThe mean duration of treatment with ETN was 53 months (mean of 44 months) and for the rest of biologics it was 43 months (mean 23 months). We estimated survival at 1, 2 and 5 years (Table 2). The Kaplan–Meier duration curve for ETN was greater than for all other biological treatments together (Fig. 1). Statistical tests of comparison between the two groups revealed significant differences (log-rank: P=.024, Breslow P=.068, Tarone-Ware P=.040).
Comparing duration between genders using ETN, there were no significant differences. No differences were found when comparing the diagnoses of RA and SA (results not shown).
DiscussionClinical trials are the gold standard of clinical evidence, but have certain limitations. They often include a limited number of patients, and have strict selection criteria such as exclusion of comorbidities, and short follow-up periods.9 However, observational registries offer a real and ongoing assessment of the drugs studied and lead to a greater knowledge on their effectiveness and long-term safety. Local registries of drugs offer a detailed look at what happens in the daily follow up. In clinical practice, adverse events, lack of primary response and secondary lack of efficiency are common problems. Even first time use of ETN did not reach a satisfactory response in 40%–50% of patients with RA and is often without effectiveness over time.10
ETN is a fully human soluble TNF receptor, whereas INF and ADA are monoclonal antibodies against TNF (INF is chimeric and ADA fully human). Its pharmacokinetics is different in some respects. Specifically, the elimination half-life is lower and ETN neutralizing antibody formation is rare.11 In addition, ETN has demonstrated efficacy in the treatment of inflammatory bowel disease whereas anti-TNF monoclonal antibodies have not done so.12,13 Furthermore, it appears that there is less incidence of TB with ETN than INF and ADA.14–17 This makes it likely that the efficacy and safety data are not identical between the three anti-TNF agents.
In our group of patients, treatment with ETN showed longer duration than the rest of biological therapy as a whole. This difference was significant from the second year of treatment onward, reaching statistical significance in the fifth year. These data are comparable with that reported by national RA registries in Germany5 (70% duration during the first year), Denmark3 (56% the second year), Italy, Lombardy18 (62.5% at 3 years) and differ from the Swedish registry4 (70% in the fifth year).
Our study found no duration differences in treatment with ETN or the rest of biologics by comparing patients with RA and AS. This differs from that reported by the Czech and BIOBADASER registries, describing increased duration of anti-TNF therapy compared with RA, although the Czech registry only includes patients who received biologics for the first time.19,20
However, our study has limitations. The main one is the limited number of patients, which may influence its statistical power, in order to not to diminish the number, the treatments were included regardless of biologics used previously, and we know that this determines the effectiveness.21 Moreover, using data from a previous registry, we were unable to include variables of clinical activity, disease duration or previous drugs, which would have been desirable in order to compare the different drugs and avoid selection bias. In addition, our data is from patients with various diagnoses and the effectiveness of biologics may be different for each disease.22
Our study, consistent with other registries, shows a lower discontinuation rate than the rest of ETN based biologic therapy. This could be due to a lower frequency of adverse events and / or better clinical efficacy. However, the study design is inappropriate to make direct comparisons and this may only be tested with randomized clinical trials.
Finally, in our case, the local registry has been useful to standardize the management of patients with biologic treatment and alert on the occurrence of adverse effects.
In summary, the data from our registry show a higher local duration of ETN treatment as compared with the rest of biologic therapy, similar to what is seen in other national registries.
Conflict of InterestThe authors declare no conflicts of interest.
Thanks to Dr. Loreto Carmona for her cooperation in drafting the article; without her enthusiasm we would have been unable to disclose the results of our modest research.
Please cite this article as: Senabre-Gallego JM, et al. Duración del tratamiento con etanercept y razones de discontinuación en una cohorte de pacientes con patología reumática. Reumatol Clin. 2011;7(6):385–8.