To evaluate the association between weight loss and changes in disease activity in patients with psoriatic arthritis (PsA).
MethodsWe performed a systematic review of the literature, with searches in Medline, Embase and Cochrane Central Library from inception until April 2015. Inclusion criteria: (1) randomized controlled trials (RCT); (2) PsA patients; (3) interventions were any intervention aimed at weight control; and (4) a PsA activity-related outcome measure was evaluated. Risks of bias were assessed by the Cochrane Collaboration scale.
ResultsOf the 215 articles identified, only 2 RCT met the inclusion criteria, 1 in abstract format. Both showed moderate risk of bias. Patients who managed to lose weight-by any method-had better results in terms of activity and inflammation. The percentage of weight loss correlated moderately with changes in inflammatory outcomes.
ConclusionWeight loss in PsA could be associated with less inflammation; however, the evidence to support this is limited.
Evaluar la asociación entre la pérdida de peso y cambios en la actividad en pacientes con artritis psoriásica (APs).
MétodosSe llevó a cabo una revisión sistemática en Medline, Embase y Cochrane Central desde el inicio hasta abril del 2015. Criterios de inclusión: 1) ensayos clínicos aleatorizados controlados (ECA); 2) pacientes con APs; 3) cualquier intervención encaminada al control de peso, y 4) evaluación de la actividad de la APs. Se evaluaron los riesgos de sesgos según la escala Cochrane.
ResultadosDe 215 artículos identificados, solo 2 ECA cumplieron los criterios de inclusión, uno de ellos en formato abstract. Ambos tenían riesgos de sesgos moderados. Los pacientes que lograban perder peso, por cualquier método, mostraban menores niveles de actividad e inflamación. El porcentaje de pérdida de peso correlaciona moderadamente con cambios en medidas de inflamación.
ConclusiónLa pérdida de peso en APs podría asociarse a menor inflamación, si bien la evidencia que lo apoya es limitada.
The prevalence of overweight and obesity in persons with psoriasis and psoriatic arthritis (PsA) is greater than that of the general population.1,2 In turn, it has been seen that obesity can be a risk factor for the development of psoriatic disease,3–6 as well as for greater disease activity and severity. This could be explained by the proinflammatory state provoked by the accumulation of adipose tissue, with changes in the expression of cytokines, such as tumor necrosis factor (TNF) α, interleukin (IL) 6 and adipokines (leptin, adiponectin).7–10 Patients with psoriasis who are also obese have a very serious skin disease and have a poorer response to treatments.11,12 In patients with PsA, obesity also predicts a poor joint response to treatment, whether or not the latter was biological.13,14 It has been found that caloric restriction reduces the levels of inflammatory cytokines in obese individuals15,16 and can considerably improve psoriasis17; however, its effect on arthritis is not clear. In the attempt to support a series of recommendations for the management of PsA, we decided to conduct a systematic review, the purpose of which was to evaluate the effect of weight loss on disease activity and the response to treatment in PsA patients.
MethodsWe carried out a systemic literature review. For this we designed a search strategy in the bibliographic databases MEDLINE (from 1960), EMBASE (from 1980) and the Cochrane Central Library from inception to April 2015. The search included MeSH terms and free text (Table 1). The search was limited to studies involving humans and to studies published in English and Spanish. Moreover, a manual search was performed using the bibliography of the included articles and from rheumatology meetings held in the United States and Europe over the preceding 2 years.
Search Strategy.
| #1 “psoriatic arthritis” |
| #2 “psoriatic arthritis”/exp |
| #3 #1 OR #2 |
| #4 “diet” |
| #5 “weight control” |
| #6 “weight reduction” |
| #7 “weight reduction”/exp |
| #8 “antiobesity agents” |
| #9 “antiobesity agents” |
| #10 “anti obesity agents” |
| #11 “metformin” |
| #12 “sibutramine” |
| #13 “orlistat” |
| #14 “xenical” |
| #15 “meridia” |
| #16 “glucophage” |
| #17 “low calorie diet” |
| #18 “low fat diet” |
| #19 nutrition$ AND (intervention$ OR education$) |
| #20 diet$ AND (intervention$ OR education$) |
| #21 “weight loss” AND (intervention$ OR education$) |
| #22 weight AND reduction |
| #23 “weight reduction”/exp |
| #24 (#4 - #23) OR |
| #25 #3 AND #24 |
Two reviewers (RA and LC) independently examined the titles and abstracts of the articles retrieved for the selection criteria. We then recorded the data of the selected studies.
We included only: (1) randomized controlled trials (RCT), in which (2) the population to be treated consisted of PsA patients; (3) the intervention was any measure aimed at weight control (diet, exercise, prescribed drugs, surgery, etc.); (4) the latter be compared with a group in which no intervention or control was introduced; and (5) the implementation of some measure of evaluation of the activity, either peripheral (66/68 joint count, Disease Activity Score in 28 joints [DAS28], DAS, visual analogue scale (VAS), enthesitis index) or axial (Ankylosing Spondylitis Disease Activity Score, Bath Ankylosing Spondylitis Disease Activity Index, Bath Ankylosing Spondylitis Functional Index, Bath Ankylosing Spondylitis Metrology Index), acute-phase reactants (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) or imaging, or other efficacy scores documented in the article.
All of the articles obtained were reviewed in detail with the aid of ad hoc designed data collection sheets. We gathered all the data on the description of the sample and on the specific results of weight control on inflammatory activity. We likewise evaluated the risk of biases using the domains proposed by the Cochrane Collaboration.18
We performed a qualitative analysis of the information collected for type of study and the population being studied, for quality and specific results, as well as a qualitative evaluation of the heterogeneity.
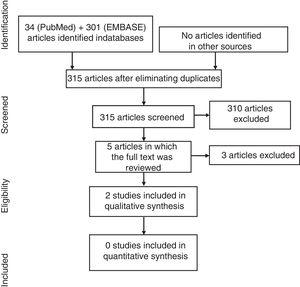
ResultsIn the diagram in Fig. 1 we describe the results of the search. In the search strategy, we identified a total of 215 articles, 5 of which were selected for a detailed review after the exclusion of 210 references on the basis of their title and abstract. Finally, 3 articles were excluded (Table 2 specifies the excluded studies and the reasons for their exclusion).
Excluded Studies and Reasons.
| Author, year | Reason for exclusion |
|---|---|
| Schett et al., 2014 | Does not analyze the effect of weight control. It is a 24-week randomized controlled trial that evaluates the impact of weight and baseline body mass index on the clinical response (ACR20 and HAQ) to apremilast in PsA patients, rather than a change in body weight |
| Di Minnet et al., 2012 | Abstract submitted to a congress. It evaluates weight reduction through diet, to reach the minimal activity in overweight/obese PsA patients, who began treatment with anti-TNF. It was excluded because it was subsequently published in 2014 and is included in the present systematic review |
| Sullivan et al., 2013 | A 24-week open trial that analyzes the effect of GLP-1 (liraglutide) on disease activity in 15 patients with inflammatory arthritis (11 with rheumatoid arthritis and 4 with PsA) and type 2 diabetes mellitus. It does not provide separate data and the sample size is too small |
ACR20, 20% improvement in American College of Rheumatology criteria; GLP-1, glucagon-like peptide; HAQ, Health Assessment Questionnaire; PsA, psoriatic arthritis; TNF, tumor necrosis factor.
Table 3 shows the data on the 2 articles that were ultimately included in the analysis.13,19 There were 2 RCT, 1 of which had the format of an abstract. The risks of biases are moderate in the study by Di Minno et al. and high in that of Abou-Raya et al., in the latter due to lack of information.
Characteristics of the Included Studies.
| Article | Design | Patients | Intervention | Activity measurements | Randomized sequence | Blinded participants | Blinded assessors | Missing outcome measures | Selective communication |
|---|---|---|---|---|---|---|---|---|---|
| Di Minno et al., 2014 | 6-month single-blind RCT | No.=126 | Hypocaloric diet group Free-managed diet group | TJC, SJC, PASI, VAS pain, HAQ, ESR and CRP | |||||
| Abou-Raya et al., 2014 | 12-month single-blind RCT | No.=55 Adults with PsA and BMI ≥30 | Control=standard treatment Diet+standard treatment Exercise+standard treatment Diet+exercise+standard treatment | ACR20, HAQ, BDI, fatigue, PASI, PGA, DAS28-CRP, high-sensitivity CRP, IL-6. IL-17 and TNF |
The colors indicate the adaptation of certain domains of the risk scale.
ACR20, 20% improvement in American College of Rheumatology criteria; BDI, Beck Depression Inventory; BMI, body mass index; CRP, C-reactive protein; DAS28, Disease Activity Score in 28 joints; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; PASI, Psoriasis Area Severity Index; PGA, Physician's Global Assessment; PsA, psoriatic arthritis; SJC, swollen joint count; TJC, tender joint count; TNF, tumor necrosis factor; VAS, visual analogue scale.
In the study of Di Minno et al.,13 patients with PsA and obesity were randomized to receive a hypocaloric diet based on an intake of fat of 30%–35% of the total daily intake and an increase in fiber and fish to at least one day a week, or a free diet, receiving certain nutritional advice. At the same time, and given the lack of symptomatic control, the patients, who had not taken biological therapy before commencing the study, began treatment with anti-tumor necrosis factor (TNF) drugs. In all, 58.7% lost weight (≥5%), which occurred more frequently in the hypocaloric diet group (49 of 63) than in the free diet group (25 of 63; P<.001). Both diets resulted in the achievement of a significant improvement in joint counts, in enthesis, and in the Psoriasis Area Severity Index (PASI), Health Assessment Questionnaire (HAQ), VAS, CRP and ESR at 6 months. The patients who lost more than 5% of their weight more frequently had a minimal disease activity, according to Coates and Helliwell20 (50%), than those who lost less weight (23%; odds ratio=4.20; 95% confidence interval, 1.82–9.66; P<.001).
The objective of the study by Abou-Raya et al.19 was to evaluate whether weight loss achieved by exercise or diet, or by both, was more effective than standard treatment alone (nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, anti-TNF-α) in improving the symptoms (pain, physical function, fatigue, depression) and systemic inflammation in obese adults with PsA. The mean reduction in body weight was 15% in all of the intervention groups versus 2% in the control group (P<.001). In the exercise group, there was a significant improvement (20%) in the American College of Rheumatology criteria (ACR20), HAQ, Beck Depression Inventory, fatigue, PASI and Physician's Global Assessment (PGA), and in the diet group there was a significant improvement in ACR20 and PASI 75 response (75% reduction) and certain significant reductions in systemic inflammatory markers. In the diet and exercise group, there was a significant improvement in ACR20, HAQ, depression, fatigue, DAS28-CRP, PASI and PGA, together with significant reductions in the serum levels of high-sensitivity CRP, IL-6, IL-17 and TNF in comparison with controls. There was a moderate correlation between the percentage of weight loss and the reduction of PASI (r=0.587).
DiscussionA number of studies have reported an elevated prevalence of cardiovascular risk factors, such as hypertension, obesity, diabetes mellitus and dyslipidemia, in PsA patients.21–27 Moreover, evidence indicates that obesity, insulin resistance, psoriasis and PsA may share a common predisposition in terms of low-grade inflammation.24 It has also been found that body mass index is associated in PsA with a poorer response to treatment.28 What was not that clear is whether weight reduction could have an effect on activity. In this systematic review we found 2 studies that support this hypothesis, 1 with a high level of evidence and another with a level somewhat lower, as it was only in the format of an abstract at that time.
There are close links between the metabolic system and the immune system.29 For example, IL-6 is a key proinflammatory cytokine that can stimulate the hypothalamus, which is associated with central obesity, hypertension and insulin resistance, and induces the production of CRP.30 Moreover there is evidence that caloric restriction reduces the levels of CRP and TNF-α15 and, thus, patients whose dietary intake is 1500 calories/day can show decreases in CRP levels for this reason, and not only for a reduction in inflammatory activity. This could explain the effect observed on the disease activity in these studies.
Other factors could have an effect on weight and on activity. For example, in the study published by Di Minno et al.,13 20% of the patients were receiving chronic treatment with oral hypoglycemic agents, and the effect on body weight that can be induced by glucagon-like peptide-1 agonists, metformin and sulfonylurea is well known; moreover, treatment was begun with an anti-TNF agent. Although the patients were taking stable doses of hypoglycemic drugs and all initiated anti-TNF therapy, it is not possible to rule out that the effect of body weight on disease activity would in part be due to those other treatments. It could even be considered that the patients that most firmly adhered to diet or exercise would be more adherent to the treatments.31
Our review was limited by the small number of published studies and by their intermediate quality. The hypothesis that weight loss in PsA could be linked to a reduction in disease activity, although indicative, is only based on 2 randomized studies, 1 of them presented in the form of an abstract. Thus, we conclude that this should be investigated in greater depth.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe present report was financed by MSD.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Almodóvar R, Zarco P, Otón T, Carmona L. Efecto de la pérdida de peso en la actividad en artritis psoriásica: una revisión sistemática. Reumatol Clin. 2018;14:207–210.