To evaluate the effectiveness and safety of tocilizumab (TCZ) monotherapy in biologic-naïve patients with rheumatoid arthritis (RA) versus patients with previous biologic exposure in a real-world setting.
Materials and methodsNon-controlled clinical-trial, 32-week prospective multicenter study including RA patients with moderate-severe disease activity starting TCZ in monotherapy who had a prior inadequate response or were intolerant to methotrexate (MTX). Effectiveness according to EULAR response evaluated at 24-week and safety at 32-weekwere assessed.
ResultsOf the 93 were enrolled of whom 84 (90%) were eligible for the effectiveness analysis. Biologic-naïve patients (n=46, 54.8%) were younger (51.5 versus 57.9) with shorter disease duration (6.4 versus 13.3) but presented similar comorbidities in comparison with non-naïve patients. DAS28 remission was achieved in a higher percentage in the group of patients with prior biological treatment. 89 adverse events (AE) were recorded in 50 patients, most of them non-serious AE (non-SAE) (86.3%).
ConclusionsIn a real world setting, TCZ exhibit similar effectiveness and safety in monotherapy in patients with RA regardless previous exposure to other biologic therapies. This study provides additional and valuable real-world findings on the use of TCZ in patients with RA.
Evaluar la efectividad y seguridad de la monoterapia con tocilizumab (TCZ) en pacientes con artritis reumatoide (AR) sin tratamiento biológico en comparación con pacientes con exposición previa a biológico en un entorno real.
Materiales y métodosEnsayo clínico no controlado, estudio multicéntrico prospectivo de 32 semanas que incluyó pacientes con AR con actividad de la enfermedad moderada-grave que comenzaron con TCZ en monoterapia y que tuvieron una respuesta inadecuada previa o fueron intolerantes al metotrexato. La eficacia de acuerdo con la respuesta EULAR fue evaluada a las 24 semanas y la seguridad a las 32 semanas.
ResultadosDe los 93 pacientes seleccionados, 84 (90%) fueron elegibles para el análisis de efectividad. Los pacientes sin tratamiento biológico previo (n=46, 54,8%) eran más jóvenes (51,5 frente a 57,9 años), con una duración más corta de la enfermedad (6,4 frente a 13,3 años), pero presentaban comorbilidades similares en comparación con los pacientes con tratamiento previo. La remisión de DAS28 se logró en un mayor porcentaje en el grupo de pacientes con tratamiento biológico previo. Se registraron 89 eventos adversos en 50 pacientes, la mayoría de ellos no graves (86,3%).
ConclusionesEn un entorno del mundo real, TCZ exhibe una eficacia y seguridad similares en monoterapia en pacientes con AR, independientemente de la exposición previa a otras terapias biológicas. Este estudio proporciona hallazgos adicionales y valiosos en el contexto del mundo real sobre el uso de TCZ en pacientes con AR.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease, characterized by chronic inflammation of joints with a significant impact on patients’ quality of life, physically, socially, psychologically and economically.1,2
The disease-modifying antirheumatic drugs (DMARDs) and biological agents against inflammatory cytokines have dramatically changed the managements of patients with RA and nowadays it is recommended to treat patients to achieve clinical remission.3,4 Methotrexate (MTX) is still the anchor drug for RA treatment.5 However, a high proportion of patients may not respond adequately, present side effects6 or poor tolerability, regardless of the dose,7 requiring additional o different therapy. Recent clinical trials have shown that treatment with anti-TNF biologics, with or without MTX, can lead to clinical remission in around 50% of patients; however the remaining half of the patients need to switch to other medications.8,9
Tocilizumab (TCZ), a biological agent targeting the IL-6 receptor has been approved for use in patients with RA.10 TCZ can be used in combination with MTX or in monotherapy, and has been proven to be beneficial in decreasing disease activity, preventing structural damage and improving function in RA patients in multiple randomized clinical trials (RCT).11 It appears that TCZ present a better safety profile when used in monotherapy that in combination with MTX.12
Data from previous RCT have proved efficacy and safety of TCZ and additional data from cohorts have confirmed this results in real world. However, additional analysis to evaluate the effectiveness of TCZ in patients in which MTX can not be prescribed due to intolerance, side effects, or adherence problems may be of interest. To the best of our knowledge, there is no reports evaluating TCZ effectiveness and safety in this specific population. Our objective is to evaluate the effectiveness and safety of TCZ in monotherapy in biologic naïve RA patients versus patients with previous biologic exposure.
MethodsStudy designMOZART (non-controlled clinical trial to evaluate the effectiveness of TCZ in patients with moderate to severe RA and in candidates for a biologic in monotherapy) (EudraCT: 2013-004051-20, and ClinicalTrials.gov: NCT02087696). This is a 32-week prospective non-controlled multicenter study to assess the effectiveness and safety of intravenous (iv) TCZ monotherapy in MTX-intolerant RA patients with moderate-severe activity.
PatientsAll RA patients included in this study fulfilled the ACR classification criteria. Patients recruited from 20 rheumatology units in Spain between June 2014 and November 2016 were consecutively registered into this study and were followed during 24 weeks. Inclusion criteria were: moderate to severe RA (DAS28≥3.2), inadequate clinical response to a stable dose of synthetic DMARDs or failure to respond to one or two biologics for a period ≥8 weeks, oral glucocorticoids (GCs) ≤10mg prednisone or equivalent with a stable dose for at least one month prior to TCZ treatment.
RA patients were enrolled if had intolerance, contraindication or lack of adherence to MTX. The intolerance to MTX was evaluated using the UKU Side Effects Rating Scale.13 Lack of adherence to MTX was evaluated using the Morisky Green Levine Test.14 Patients were excluded if presented a hepatitis C virus infection, active diverticulitis, latent tuberculosis (positive PPD or suspicious chest X-ray), women pregnant or breastfeeding. Additional information regarding selection criteria are available in the Supplementary Material section (Table 1S).
Enrolled patients, who had MTX stopped 4 weeks before the baseline visit, were scheduled for eight visits (weeks 0, 4, 8, 12, 16, 20, 24, and a visit at week 32 to evaluate safety).
All enrolled patients were offered to initiate open-label iv treatment with TCZ 8mg/kg in monotherapy per protocol every 4 weeks for 6 months from June 2014 to April 2017. Patients who presented abnormal laboratory parameters (liver enzymes, absolute neutrophil counts and/or platelet counts) during treatment with TCZ, had their doses adjusted in accordance with the TCZ drug information sheet.
Demographic data, including date of diagnosis, comorbidities, current and previous treatments for RA were collected from the medical charts. The following parameters were evaluated at 0, 4, 8, 12, 24 and 32 weeks: tender joint count (TJC) 28, swollen joint count (SJC) 28, patient's global assessment (Pt-GA) of disease activity, physician's global assessment (Ph-GA) of disease activity, ESR, CRP, Health Assessment Questionnaire (HAQ), Short Form 36 Health Survey (SF-36), and fatigue by the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Disease activity was assessed by the Disease Activity Score (DAS28), the Clinical Disease Activity Index (CDAI) and the Simple Disease Activity Index (SDAI), which were calculated according to their formula.
The primary endpoint was the EULAR response after 24 weeks of TCZ treatment. The EULAR response criteria classify patients as good, moderate, or non-responders using the individual amount of change determined by the DAS28-ESR and the DAS28-ESR value (low, moderate, or high) and can also be applied using the DAS28-ESR (Table 2S, Supplementary Material).15,16
Secondary endpoints were: (1) effectiveness of TCZ after 24 weeks according to the American College of Rheumatology 20%, 50%, and 70% improvement (ACR 20/50/70) criteria; (2) to compare response to TCZ in monotherapy according to previous treatment with biologics; and (3) to assess the safety of TCZ by adverse events reported by patients or recorded by researchers.
This study was developed in accordance with the protocol and guidelines of Good Clinical Practice and was carried out following the principles outlined in the Helsinki Declaration (v.2013). The study protocol was approved by the Ethics Committee for Clinical Research of the Hospital University Vall d’Hebron, Barcelona, Spain (Approval number: ID-RTF010). All patients provided written informed consent to participate in the study and to publish the data obtained.
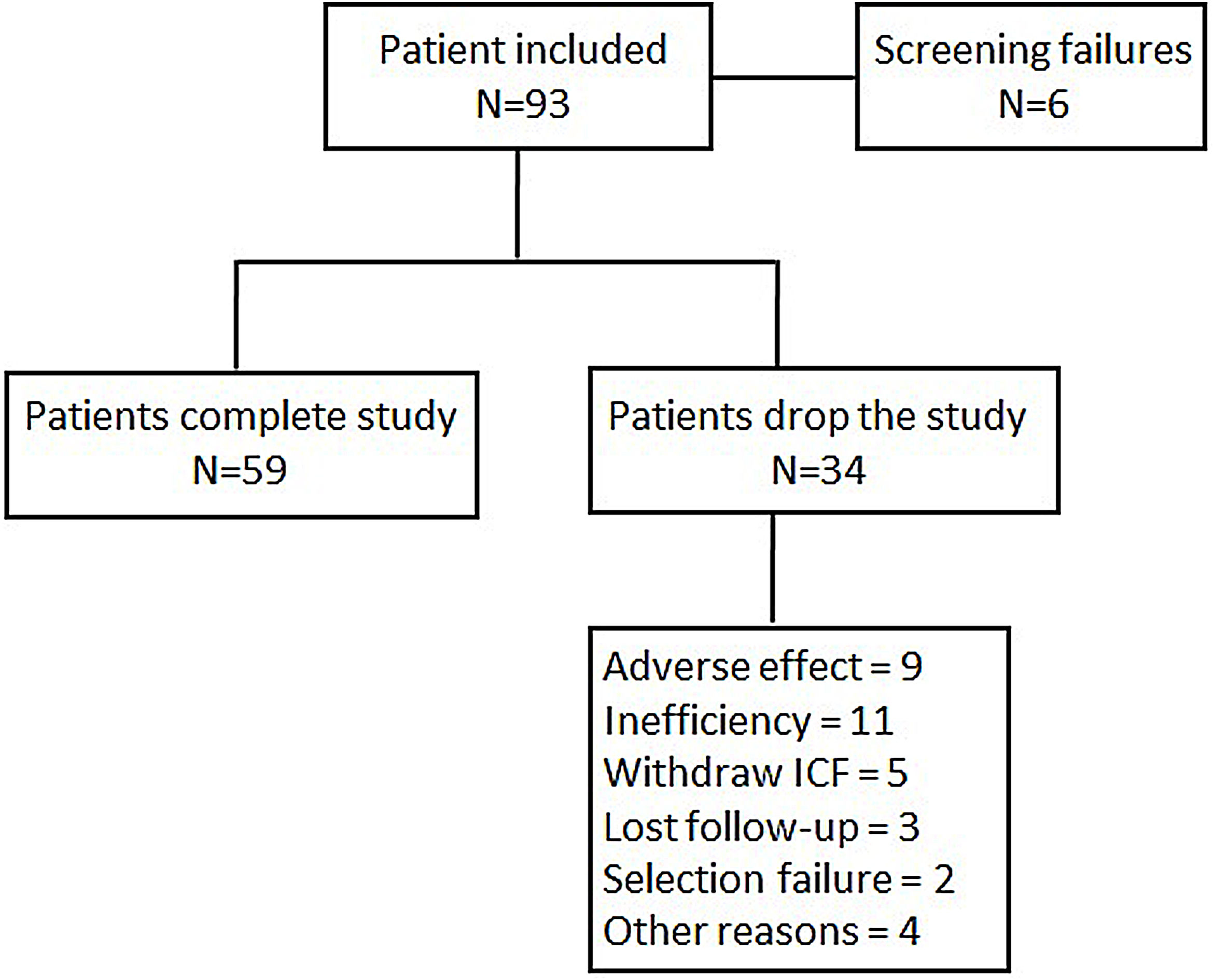
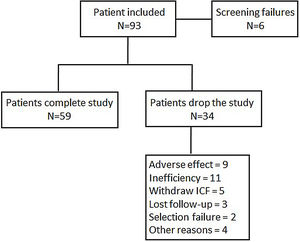
Statistical analysisIn order to obtain an estimation whereby 78% of patients achieved a moderate or good EULAR response, with 10% accuracy and a 95% confidence interval, it was necessary to include 109 patients in the study. By assuming a loss rate of 10%, the total number of recruited patients needed to achieve the primary objective was 122. When 93 patients were recruited, the statistic power to reach 78% of a good or moderate EULAR response was 0.76, at which point the scientific committee halted further inclusion. A flow chart of patient inclusion is shown in Fig. 1.
To evaluate one of the secondary objectives, patients were stratified into two cohorts: cohort A, patients naïve to biologics (n=50); and cohort B, patients who previously received 1 or 2 biologics for RA treatment (n=43).
An analysis of frequency of qualitative and quantitative variables was performed. Qualitative variables were expressed in percentage and absolute values, and quantitative variables were expressed as a means plus standard deviation.
Effectiveness of TCZ treatment in monotherapy was assessed based on the percentage of patients who achieved moderate and good EULAR responses after 24 weeks of treatment.
An intention-to-treat analysis was conducted. If a patient terminated treatment before week 24, he/she was evaluated as a responder or non-responder according to the results obtained from the last registered visit.
To assess the effectiveness of reducing the DAS28-ESR, the median score at baseline was compared to that at week 24 using the two-sided Student t-test for related samples. In order to evaluate whether patients in cohorts A and B could obtain different effectiveness results, an ANCOVA was performed.
All patients receiving at least one TCZ dose were included in the safety analysis. A description of the AEs recorded throughout the study period was performed.
IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. was used to conduct the statistical analyses.
ResultsBaseline characteristics of patient populationA total of 93 were enrolled of whom 84 were eligible for the effectiveness analysis. Table 1 summarizes the sociodemographic characteristics of the patients. The mean age of patients included was 54.4 years (SD: 12.8) with 83.3% of the participants being women. 46 (54.8%) patients were naïve to biological. Patients naïve to biologics were older than those previously treated with these compounds (57.9±12.5 vs 51.5±12.4 years), albeit with shorter disease durations (6.4±6.6 vs 13.3±11.5 years). Among the patients included, 77 (91.7%) were intolerant to MTX, 4 (4.8%) had contraindications to this drug and 1 (1.2%) showed a lack of adherence to MTX. The DAS28 at the entrance to the study was 5.5±1.1, with no differences between biologic naive and non-naive patients. Other RA activity indexes such as CDAI or SDAI, the counts of tender and swollen joints, acute phase reactants or functional capacity as assessed by HAQ did not reveal any differences between biologic naïve and non-naïve patients. Most patients (78.6%) had been receiving corticosteroids treatment at doses lower than 10mg/day of Prednisone or equivalent. The previous use of biologics and disease activity at study enrollment did not influence the percentage of patients with intolerance, contraindications or lack of adherence.
Baseline sociodemographic and clinical characteristics of the population included in the analysis of study.
| Variables | Total (84) |
|---|---|
| Sex, female, n (%) | 70 (83.3) |
| Age (years), mean (SD) | 54.4 (12.8) |
| Age by group (years) | |
| Naive patients, mean (SD) | 51.5 (12.4) |
| Non naive patient, mean (SD) | 57.9 (12.5) |
| Disease duration, years, mean (SD) | 9.5 (9.7) |
| Disease duration by group (years) | |
| Naive patients, mean (SD) | 6.4 (6.6) |
| Non naive patient, mean (SD) | 13.3 (11.5) |
| N. previous synthetic DMARDs | |
| 0 n (%) | 2 (2.4) |
| 1 n (%) | 15 (17.9) |
| >1 n (%) | 67 (79.8) |
| Naive patients, n (%) | 46 (54.8) |
| DAS28-ESR, mean (SD) | 5.5 (1.1) |
| CDAI, mean (SD) | 30.9 (11.1) |
| SDAI, mean (SD) | 36.2 (17.1) |
| SF36 (0–100), mean (SD)) | 90.7 (6.8) |
| FACTI-F, mean (SD) | 24.5 (9.7) |
| CRP, mg/L, mean (SD) | 13.2 (17.1) |
| ESR, mm/1sthour, mean (SD) | 33.4 (29.1) |
| HAQ, mean (SD) | 1.8 (0.7) |
| Hypertension, n (%) | 29 (34.5) |
| Diabetes, n (%) | 6 (7.1) |
| Dyslipidemia, n (%) | 19 (22.6) |
| Solid neoplasms, n (%) | 1 (1.2) |
| Cerebrovascular accident, n (%) | 3 (3.6) |
| Lung disease, n (%) | 3 (3.6) |
| Chronic renal failure, n (%) | 5 (6.0) |
| BMI, kg/m2, mean (SD) | 27.0 (5.3) |
| Extraarticular manifestations, n (%) | 9 (10.7) |
| NSAID, n (%) | 45 (53.6) |
| GC, n (%) | 66 (78.6) |
All variables are recorded at the time of inclusion in the study. Categorical variables are expressed as number (n) and percentages (%); SD: standard deviation. BMI: body mass index; CDAI: Clinical disease activity index; CRP: C-Reactive Protein; DAS28-ESR: Disease Activity Score using 28 joints-erythrocyte sedimentation rate; DMARD: Disease-modifying antirheumatic drugs; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; GC: glucocorticoids; HAQ (0-3): Health Assessment Questionnaire; MTX: Methotrexate; NSAID: non-steroidal anti-inflammatory drugs; SDAI: Simple disease activity index; SF36: Short Form-36.
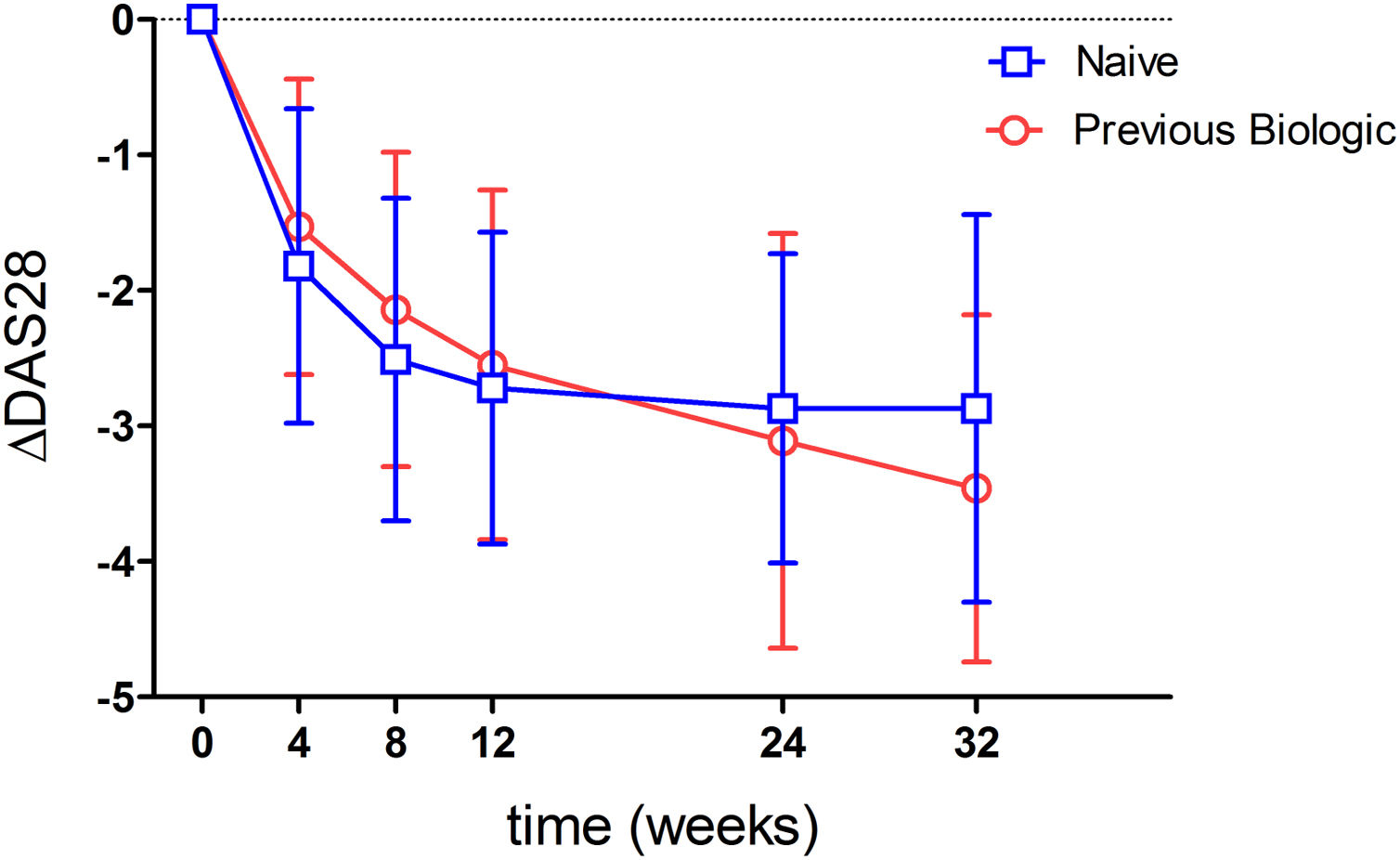
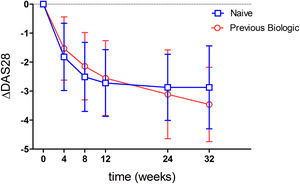
Ninety-three point eight percent of the patients analyzed had a good or moderate EULAR response. When stratifying by previous biologics treatment, a numerically higher percentage of naive patients showed a response (95.5%) compared to those with prior biologics use (91.7%) (p=0.53). When analyzing effectivenes according to moderate or good response, most patients showed a moderate response (Tables 2 and 3). This was true when analyzed either globally (92.5%) or separately by the previous use or non-use of biologics (93.2% vs 91.7%) (Table 3). Fig. 2 shows the mean variation in DAS28-ESR throughout the study with respect to baseline, which proved similar between patients naïve to biologics and those previously treated with biologics. An analysis has been carried out to evaluate the effectiveness of Tocilizumab, separating the results of the response variables by weeks to differentiate patients naïve to biological therapy versus patients who received prior treatment with biological therapy and where it can also be seen that naive patients had in every week a higher percentage of patients with EULAR response (Table 4). Fifty-eight point five percent of the naïve to biological patients reached remission and thirty-eight point 2 percent of the patients with previous biological therapy (Table 3). The rates of ACR response criteria are available in Table 2. TCZ was discontinued in 11 patients due to inefficacy and in the Table 5 also shows the causes of withdrawals of the patients and the mean time until leaving the study.
Efficacy of Tocilizumab treatment in monotherapy after 24 weeks of treatment.
| Variables | Week | p-value (per week) | ||||||
|---|---|---|---|---|---|---|---|---|
| 4 wks | 8 wks | 12 wks | 24 wks | 4 wks | 8 wks | 12 wks | 24 wks | |
| No response EULAR, n (%) | 25 (31.3) | 12 (15.8) | 5 (6.9) | 7 (10.8) | <0.001 | <0.001 | <0.001 | <0.001 |
| Moderate EULAR response, n (%) | 54 (68.8) | 63 (82.9) | 66 (91.7) | 57 (87.7) | ||||
| Good EULAR response, n (%) | 1 (1.3) | 1 (1.3) | 1 (1.4) | 1 (15) | ||||
| ACR20, n (%) | 71 (88.8) | 73 (96.1) | 67 (93.6) | 58 (89.2) | 0.023 | 0.57 | 0.17 | 0.033 |
| ACR50, n (%) | 67 (83.8 | 70 (92.1) | 67 (93.1) | 57 (87.7) | 0.002 | 0.11 | 0.17 | 0.016 |
| ACR70, n (%) | 62 (77.5) | 65 (85.5) | 65 (90.3) | 55 (84.6) | <0.001 | 0.005 | 0.05 | 0.004 |
| Δ DAS28, mean±SD | −1.7 (1.1) | −2.3 (1.2) | −2.6 (1.2) | −3 (1.3) | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ% DAS28, mean±SD | −30.3 (21.1) | −42.0 (21.9) | −48.2 (23.0) | −54.0 (21.6) | <0.001 | <0.001 | <0.001 | <0.001 |
| DAS28-ESR Remission, n (%) | 14 (17.72) | 23 (31.08) | 26 (37.14) | 33 (55) | Not p-value* | |||
| CDAI Remission, n (%) | 1 (1.27) | 2 (2.63) | 5 (7.04) | 4 (6.56) | ||||
Data are expressed as number (n) and percentages (%). Efficacy of Tociluzumab treatment were assessed based on EULAR response: Delta DAS28_ESR (DAS28_ESRLast visit evaluated – DAS28_ERSVisit1). EULAR: European League Against Rheumatism; ACR: American College of Rheumatology. Remission DAS28-ESR<2.6; Remission CDAI≤2.8.
Efficacy of Tocilizumab after the end of treatment and patients in remission at their last study visit according to DAS28-ESR and CDAI criteria by naive or previous biological group.
| Variables | Total | Naive biological therapy | ||
|---|---|---|---|---|
| 84* | No | Yes | p-value | |
| Good or moderate EULAR response n (%) | 75 (93.8) | 33 (91.7) | 42 (95.5) | 0.528 |
| Good EULAR response, n (%) | 1 (1.3) | 0 (0.0) | 1 (1.3) | |
| Moderate EULAR response (n, %) | 74 (92.5) | 33 (91.7) | 41 (93.2) | |
| ACR20 response, n (%) | 49 (61.3) | 23 (63.9) | 26 (59.1) | 0.661 |
| ACR50 response, n (%) | 36 (45.0) | 14 (38.9) | 22 (50.0) | 0.320 |
| ACR70 response, n (%) | 10 (12.5) | 3 (8.3) | 7 (15.9) | 0.308 |
| DAS28-ESR Remission, n (%) | 37 (49.33) | 24 (58.54) | 13 (38.24) | Not p-value* |
| CDAI Remission, n (%) | 4 (5.26) | 4 (9.76) | 0 (0) | Not p-value* |
Data are expressed as number (n) and percentages (%). Efficacy of Tociluzumab treatment were assessed based on EULAR response: Delta DAS28_ESR (DAS28_ESRLast visit evaluated – DAS28_ERSVisit1). EULAR: European League Against Rheumatism; ACR: American College of Rheumatology. Remission DAS28-ESR<2.6; Remission CDAI≤2.8.
Efficacy of Tocilizumab treatment in monotherapy for RA patients naïve to previous biological therapy versus RA patients who received previous treatment with biological therapy.
| Variables | Naive biological therapy | No naive biological therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| Week | Week | |||||||
| 4 | 8 | 12 | 24 | 4 | 8 | 12 | 24 | |
| No response EULAR, n (%) | 13 (30.2) | 6 (15.0) | 2 (5.1) | 3 (8.1) | 12 (32.4) | 6 (16.7) | 3 (9.1) | 4 (14.3) |
| Moderate EULAR response, n (%) | 29 (67.4) | 33 (82.5) | 36 (92.3) | 33 (89.2) | 25 (67.6) | 30 (83.3) | 30 (90.9) | 24 (85.7) |
| Good EULAR response, n (%) | 1 (2.3) | 1 (2.5) | 1 (2.6) | 1 (2.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ACR20, n (%) | 39 (90.7) | 37 (92.5) | 36 (92.3) | 33 (89.2) | 32 (96.5) | 36 (100) | 31 (93.9) | 25 (89.3) |
| ACR50, n (%) | 36 (83.7) | 36 (90.0) | 36 (92.3) | 32 (86.5) | 31 (83.8) | 34 (94.4) | 31 (93.4) | 25 (89.3) |
| ACR70, n (%) | 34 (79.0) | 33 (82.5) | 36 (92.3) | 30 (81.1) | 28 (76.7) | 32 (88.9) | 29 (87.8) | 25 (89.3) |
| Δ DAS28, mean±SD | −1.8 (1.1) | −2.5(1.2) | −2.7 (1.1) | −2.8 (1.1) | −1.6 (1.2) | −2.2 (1.2) | −2.6 (1.3) | −3.2 (1.5) |
| Δ% DAS28, mean±SD | −34.0 (22.1) | −46.3 (23.2) | −51.2 (23.1) | −53.8 (22.2) | −26.2 (19.4) | −37.4 (19.7) | −44.5 (22.7) | −54.3 (21.2) |
Data are expressed as number (n) and percentages (%). Efficacy of Tociluzumab treatment were assessed based on EULAR response: Delta DAS28_ESR (DAS28_ESRLast visit evaluated – DAS28_ERSVisit1). EULAR: European League Against Rheumatism; ACR: American College of Rheumatology.
Causes of withdrawals and times of these withdrawals.
| Causes of withdrawals | Time in weeks |
|---|---|
| Selection failure, mean±SD | 16±0 |
| Inefficiency, mean±SD | 16.3±7.6 |
| Other motives, mean±SD | 17.9±8.4 |
| Lost follow-up, mean±SD | 26.2±2.0 |
| IC withdrawal, mean±SD | 13.4±8.1 |
| Treatment interruption for AE, mean±SD | 7.4±3.2 |
| Total time follow-up, mean±SD | 14.7±8.0 |
A total 89 AEs were recorded in a total of 50 patients. Eighty-six percent were non-serious and occurred mainly in biologic naïve patients. Fourteen serious AEs (SAEs) were recorded during the study; these corresponded to 6 patients, with 8 of them being drug-related SAEs. Four of these 6 patients discontinued TCZ due to serious adverse drug reactions. Three patients presented RA reactivation characterized by musculoskeletal pain and peripheral joint swelling, type IV hypersensitivity reaction, syncope, colonic abscess, rash, diverticular perforation, myelopathy, spinal stenosis, spinal osteoarthritis, vocal cord paresis, lower extremity fracture, and/or spontaneous miscarriage (Table 6). No opportunistic infections, deaths, neoplasms or major adverse cardiovascular events (MACE) were reported during the study.
Adverse events at week 32 (safety population).
| Total | Naive biological therapy | ||
|---|---|---|---|
| Number of patients with at least one AE, n (%) | 50 | 21 (42) | 29 (58) |
| Number of AE, n | 89 | 32 | 57 |
| Number of SAE, n (%) | 14 (15.7) | 1 (3.1) | 13 (22.8) |
| Patients with >1 AE, n (%) | 20 (21.5) | 7 (16.3) | 13 (26) |
| Infections and infestations | 12.6 (8.0–18.9) | 12.3 (6.4–21.6) | 12.9 (6.5–23.2) |
| Disorders of the skin and subcutaneous tissue | 6.0 (3.0–10.8) | 3.1 (0.6–9.0) | 9.4 (4.1–18.5) |
| Nervous System Disorders | 4.9 (2.3–9.4) | 1.0 (0.0–5.7) | 9.4 (4.1–18.5) |
| Metabolism and nutrition disorders | 4.4 (1.9–8.7) | 3.1 (0.6–9.0) | 5.9 (1.9–13.7) |
| Musculoskeletal and connective tissue disorders | 4.4 (1.9–8.7) | 2.1 (0.2–7.4) | 7.1 (2.6–15.4) |
| Gastrointestinal disorders | 3.3 (1.2–7.2) | 1.0 (0.0–5.7) | 5.9 (1.9–13.7) |
| Disorders of blood and lymphatic system | 2.2 (0.6–5.6) | 3.1 (0.6–9.0) | 1.2 (0.0–6.6) |
| Respiratory. thoracic and mediastinal disorders | 2.2 (0.6–5.6) | 2.1 (0.2–7.4) | 2.4 (0.3–8.5) |
| Traumatic injuries. poisonings and complications of surgical procedures | 1.6 (0.3–4.8) | – | 3.5 (0.7–10.3) |
| Immune system disorders | 1.1 (0.1–4.0) | – | 2.4 (0.3–8.5) |
| General disorders and alterations in the place of administration | 1.1 (0.1–4.0) | 1.0 (0.0–5.7) | 1.2 (0.0–6.6) |
| Eye disorders | 1.1 (0.1–4.0) | 1.0 (0.0–5.7) | 1.2 (0.0–6.6) |
| Vascular disorders | 1.1 (0.1–4.0) | 1.0 (0.0–5.7) | 1.2 (0.0–6.6) |
| Pregnancy. puerperium and perinatal diseases | 0.5 (0.0–3.1) | – | 1.2 (0.0–6.6) |
| Medical and surgical procedures | 0.5 (0.0–3.1) | – | 1.2 (0.0–6.6) |
| Cardiac disorders | 0.5 (0.0–3.1) | 1.0 (0.0–5.7) | – |
| Ear and labyrinth disorders | 0.5 (0.0–3.1) | – | 1.2 (0.0–6.6) |
| Hepatobiliary disorders | 0.5 (0.0–3.1) | 1.0 (0.0–5.7) | – |
Data are expressed Person-week rate (IC95%) per 1000. AE: adverse events; SAE: serious adverse events
In this multicenter non-controlled clinical trial, a higher percentage of patients with RA with moderate to high disease activity and non-tolerant or with contraindication to MTX showed a better response to TCZ in monotherapy when they did not have a previous treatment with biological, although these findings did not reach statistical significance. Although some studies in which TCZ was used in combination with MTX achieved a higher rate of response than TCZ in monotherapy,17,18 other reports have shown no clinically relevant superiority of adding TCZ to MTX when patients have not completed response to MTX over switching to TCZ in monotherapy.19 Our results are in line with these findings concerning effectiveness, both according to EULAR and ACR response.19–23
Effectiveness proved to be similar between patients with 1 or 2 previous biologics or in whom TCZ was the first-line biologic. These findings further support the use of TCZ as a first-line option in monotherapy, although TCZ is commonly administered as a second-line choice for patients who require biologics in monotherapy in routine care. The good results obtained with TCZ are consistent with those of other studies.
Our finding may be largely explained by the similarity between baseline characteristics for both groups not only in disease activity but also in functional status. Our findings are in contrasts with other studies, in which patients without prior exposure to biologics experienced better effectiveness probably because of less severity, refractivity and lower DAS28 at study entry.24–26
In terms of safety, only nine patients withdrew treatment due to AEs, four of them presenting SAEs. Of those SAEs reported, only 8 were directly drug related, and the remaining ones were events that occurred during the study period but non related to TCZ. The most frequent AEs were infections, as previously reported in other studies.27,28 These results indicate that attention must be paid to the onset of serious infections during TCZ treatment as well as with anti-TNF agents, but that the safety profile of TCZ is acceptable in clinical practice.
The main strength of our study is that represents results in routine care patients. Our study main limitation is the design as .a non-controlled interventional study. All patients received TCZ as monotherapy without any control group.
In conclusion, this study demonstrates effectiveness of TCZ according to significant DAS28 improvement, with no differences between naïve and previously treated with biologics patients. The percentage of patients in remission was higher in naïve patients. Our data support that TCZ in monotherapy is a highly effective treatment choice for patients with moderate and severe RA, with better remission rates in patients with no previous therapy with biologics, in clinical practice.
FundingThis study was funded by ROCHE Farma. The Spanish Society of Rheumatology is the sponsor of this study and has participated in the study design; in the analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The corresponding author had full access to all study data and had final responsibility for the decision to submit the manuscript for publication.
Conflict of interestThis work was funded by Roche, which manufactures tocilizumab.
S Marsal Barril has no conflicts of interest to declare.
MA Martin-Martinez has no conflicts of interest to declare.
FJ Blanco-Garcia has no conflicts of interest to declare.
A Fernandez Nebro has acted as a consultant for Roche and Sanofi.
R Garcia-Vicuña has recieved personal fees from Biogen, Lilly, Pfizer, Roche, Sandoz and Sanofi; grants from Abbvie, BMS, Lilly, MSD, Roche, Sandoz, Sanofi.
J Tornero-Molina has acted as a consultant for Gebro, Lilly, Pfizer and Roche.
F Sánchez-Alonso has no conflicts of interest to declare.
M Novella-Navarro declares grants from Janssen, UCB Pharma, Novartis, Lilly, Menarini, Grunhental, Nordic, Pfizer, Gebro.
A Escudero-Contreras has no conflicts of interest to declare.
JJ Alegre-Sancho has no conflicts of interest to declare.
A Urruticoechea-Arana has no conflicts of interest to declare.
MS Bustabad-Reyes has no conflicts of interest to declare.
P Trenor-Larraz has no conflicts of interest to declare.
T Perez-Sandoval has no conflicts of interest to declare.
MI Tevar-Sánchez has no conflicts of interest to declare.
JT Sánchez-Costa has no conflicts of interest to declare.
E Raya declares grants from Abbvie, Amgen, BMS, Janssen, Lilly, MSD, Novartis, Pfizer, Roche and UCB.
This project has been funded by ROCHE Farma. The design, analysis and interpretation of the results have been done independently.