Abatacept (ABA) is a recombinant human fusion protein that blocks co-stimulation signals on T lymphocytes, impeding their activation. Randomized and controlled trials examining efficacy and safety have been performed with ABA combined with methotrexate (MTX), vs MTX monotherapy and vs infliximab (IFB) combined with MTX in patients with rheumatoid arthritis and who are naïve to biologic therapy. ABA has shown to be more effective than MTX and at least as effective as IFB+MTX, in terms of activity and clinical remission, physical function and reduction in radiological progression. Safety data at 7 years have shown that the drug is comparable to MTX in monotherapy and safer than the IFB+MTX combination, although infections still constitute the main risk when using ABA. This review summarizes the safety and efficacy data of the AIM, ATTEST, Phase IIb IM101-100, and AGREE trials.
El abatacept (ABA) es una proteína de fusión recombinante humana que permite el bloqueo de la señal co-estimuladora del linfocito T, evitando su activación. Se han realizado estudios aleatorizados y controlados de eficacia y seguridad del ABA combinado con metotrexato (MTX), frente a MTX en monoterapia y frente a infliximab (IFB) combinado con MTX en pacientes con artritis reumatoide naive a terapia biológica. ABA ha demostrado ser más eficaz que el MTX y al menos igual que IFB+MTX, en términos de actividad y remisión clínica, funcionalidad física y disminución de la progresión radiológica. Los datos de seguridad a 7 años han demostrado que el fármaco es equiparable al MTX en monoterapia y más seguro que la combinación IFB+MTX, aunque las infecciones continúan siendo el principal riesgo del uso de ABA. En esta revisión se resumen los datos de seguridad y eficacia de los estudios AIM, ATTEST, fase IIb IM101-100 y AGREE.
Available epidemiological data on the Spanish Society of Rheumatology put the prevalence of rheumatoid arthritis (RA) in the Spanish population at 0.5%,1 which means that at least 200000 people are suffering from this disease in our country. The annual incidence of RA in Spain is 8.3 cases/100000 inhabitants, similar to the two neighboring countries.2 The disease affects mainly women (3:1, female/male) with a predilection for the 4th and 5th decades of life, with a long period of joint involvement and a significant increase in morbidity and mortality, which is globally increased over the general population.
In recent decades it has been suggested that RA is presenting with a more benign clinical course. However, this finding is probably due to recent advances in the knowledge of its natural history and pathogenesis, leading to a much earlier diagnosis and more aggressive treatment and not to real changes in the intrinsic aggressiveness of the disease. Regarding therapy, during the last decade the therapeutic arsenal of RA has been strongly reinforced by new biological drugs. These include abatacept (ABA), a recombinant human fusion protein consisting of the extracellular fraction of CTLA-4 receptor and the Fc domain of human IgG1. This molecule allows in vivo blocking of co-stimulation signals by preventing activation of the T lymphocyte (TL). The efficacy and safety of ABA in patients with RA have been supported by the results of randomized controlled trials in patients with RA and data from long-term follow up. This review will focus on the efficacy and safety data obtained from the AGREE, AIM, ATTEST clinical trials, and the 101-100 IM Phase IIb study in which ABA was studied in methotrexate naïve RA patients or those with an inadequate response to methotrexate (MTX) who had not received treatment with anti-tumor necrosis factor (TNF)—a agent.
Results of Clinical EfficacyAIM Study (Abatacept in Inadequate Response to Methotrexate)AIM was a double-blind, randomized, placebo-controlled, multicenter phase III, one-year parallel dose trial that included patients with active RA (No.=656) and with an inadequate response to disease modifying antirheumatic drugs (DMARDs) including methotrexate (MTX) and had never been treated with anti-TNF. The primary objectives were assessed at 6 months and then patients went to an open-label extension follow-up phase lasting 2–5 years.
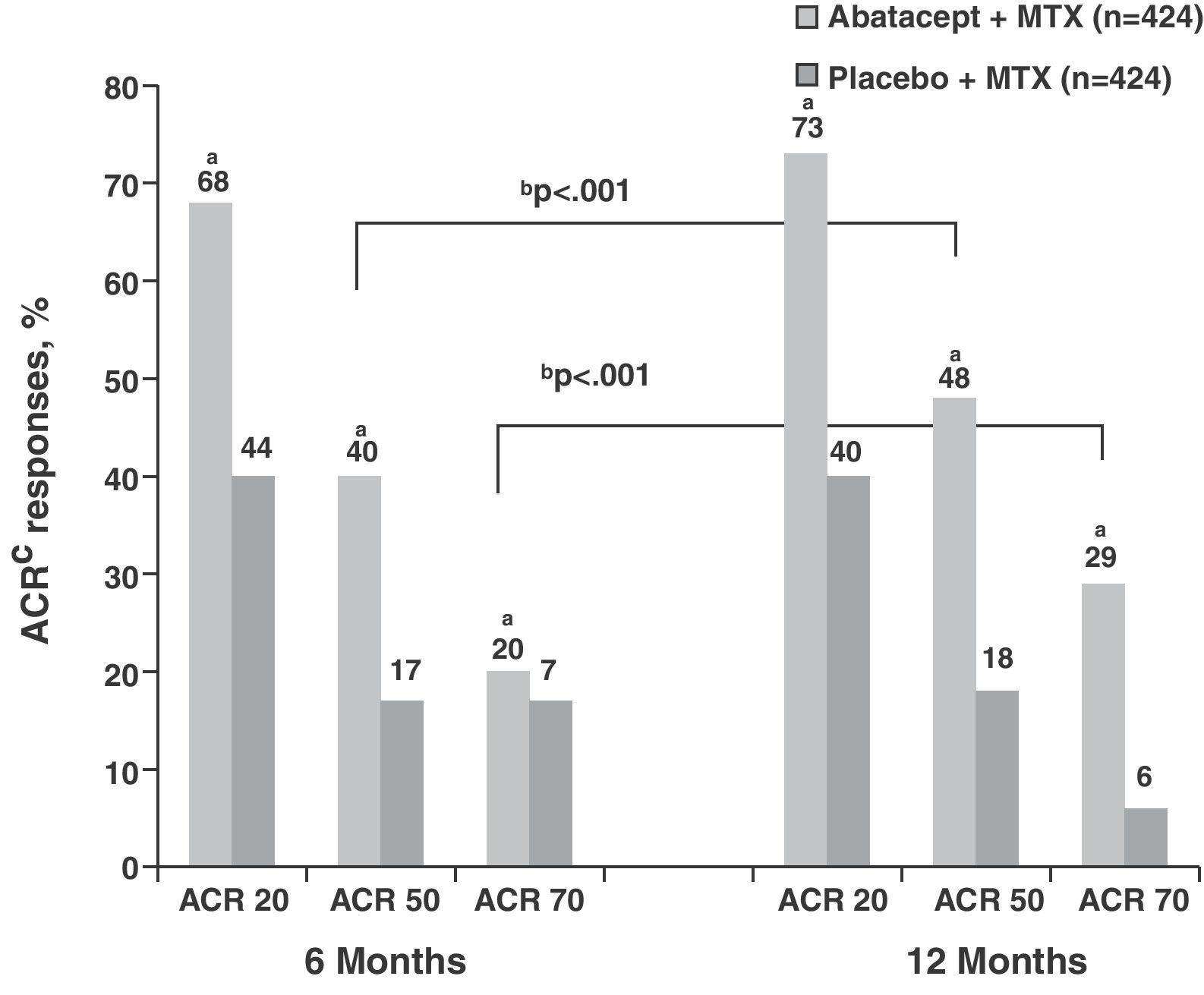
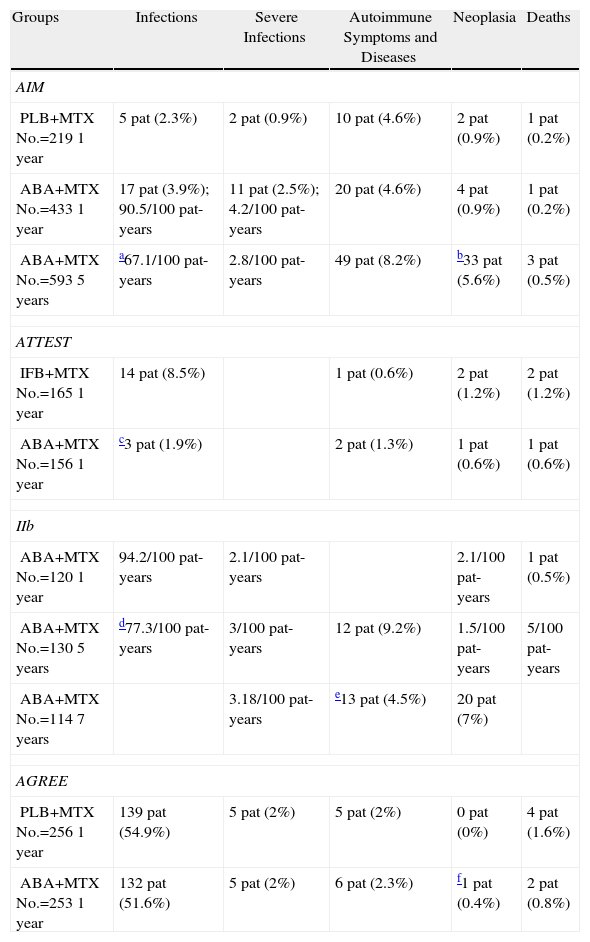
The response rates obtained by ACR and DAS28 composite indices in the ABA+MTX group (No.=433) were high even from the sixth month cutpoint compared to placebo+MTX group (PLB) (No.=219) (ACR50 39.9% vs 16.8%; ACR70 19.8% vs 6.5%, P<.001) and increased progressively each year, showing differences from the control group (ACR50 48.3% vs 18.2%; ACR70 28.8% vs 6.1%, P<.001) (Fig. 1).3 Regarding the data obtained in the assessment by DAS28 at the start of treatment both groups showed high clinical activity (mean DAS28=6.4). At 6 months these figures were reduced in the ABA+MTX group compared to control when considered either low activity (DAS28≤3.2; 30.1% vs 10.0%, P<.001) or remission (DAS28≤2.6; 14.8% vs 2.8%, P<.001). Upon completion of the study year, 44.1% were in the low activity group and more than a quarter of patients (25.4%) in the treatment arm were in remission compared to only 1.9% in the PLB+MTX group.
ACR response at 6 and 12 months in the abatacept group compared to placebo. aP<.001 for abatacept vs placebo; bP<.001 for abatacept alone, 12 vs 6 months; cITT population, in which all dropouts were considered as non responders regarding ACR after dropout. ACR: American College of Rheumatology response criteria; ITT: intention to treat; MTX: methotrexate.
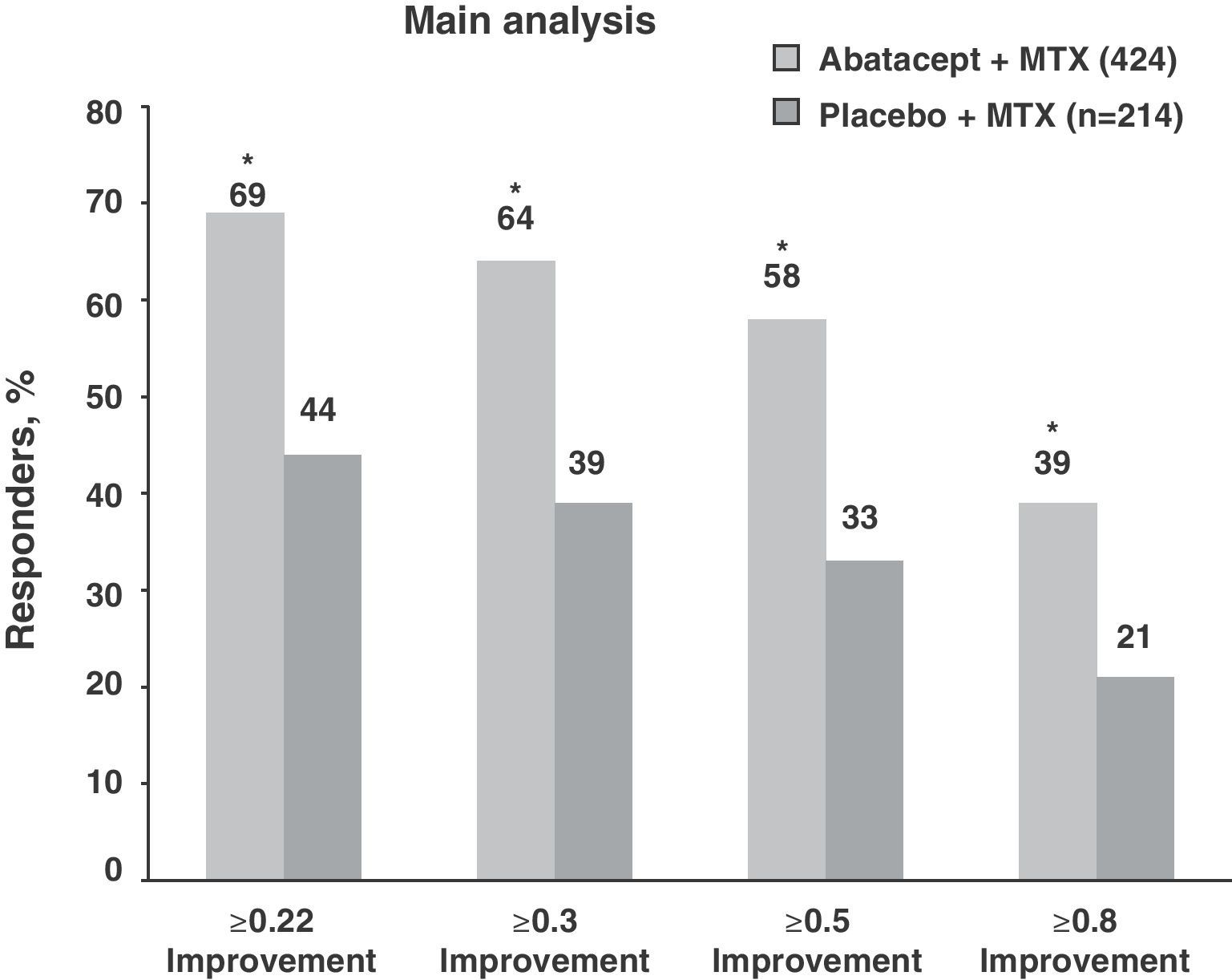
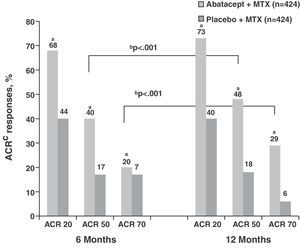
In relation to physical function, both treatment groups showed high HAQ-DI (1.7) scores at baseline. Those considered as responders achieved at least a ≥0.3units from baseline difference in the HAQ-DI. Following this criterion, the ABA+MTX group showed statistically significant improvements compared to placebo (63.7% vs 39.3%, P<.001) (Fig. 2). Moreover, there was improvement in the physical and mental components, in the quality of life test, in the sleep quality test measured by a sleep problem index (SPI) and fatigue determined by visual analog scale (VAS) during first year monitoring of patients in the ABA+MTX group with respect to the control group.3
Percentage of patients treated with abatacept who reached an HAQ-DI response at 12 months. *P<.001 for abatacept vs placebo; based on an ITT population, where dropouts considered as non responders; responders according to HAQ reached a mean baseline reduction of 0.3units. HAQ: health assessment questionnaire; ITT: intention to treat; MTX: methotrexate.
The importance of this trial lies not only in the good results obtained in response rates, but above all, radiographic progression data regarding joint damage. They evaluated structural joint damage changes from baseline with both the total Sharp score (TSS) as modified by Genant and its two components, erosion and impingement. At one year, the ABA+MTX group showed a delay in radiological progression with about half the total score compared with PLB+MTX (0.25 vs 0.53, P<.029).3
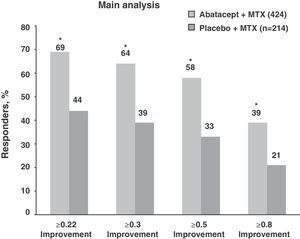
After the first year, the trial continued with an open-label extension phase lasting 2 and 5 years. At 5 years, 70.4% of patients in the ABA+MTX remained in the study. In this period, ACR figures, rather than diminishing or stabilizing, gradually increased, showing high levels of ACR50: 61.7% and 61.1% and ACR70: 38% and 39.6% and at 2 and 5 years, respectively. These percentages were stable up to 7 years after (Fig. 3). Similar results occurred with the DAS28 response during the follow-up period: DAS28≤3.2: 56.1% and 54.7% and DAS28≤2.6: 30.9% and 33.7% also at 2 and 5 years, respectively.4
In parallel with the clinical responses, there was a significant improvement in HAQ-DI and SF-36 that was maintained at 2 and 5 years.5,6
With regard to structural damage at 2 years, there was a 57% reduction of joint damage (66% reduction in erosion and impingement 47%). In subsequent years there was a continued slowing of total structural damage (0.37, 0.34, and 0.26), erosions (0.23, 0.23, and 0.11), and impingement (0.14, 0.11, and 0.14). At 5 years, 45.1% of patients remained without progression of structural damage.7
All these data confirm the clinical efficacy of abatacept, both associated with MTX and in those patients naïve to anti-TNF therapy.
The ATTEST Study (Abatacept or Infliximab vs Placebo, Tolerance Test, Efficacy, and Safety in the Treatment of RA)This was a randomized, double-blind, multicenter phase III trial comparing directly the efficacy of two biologic therapies, ABA (10mg/kg/4 week, No.=156) and infliximab (IFB) (3mg/kg/8 week, No.=165) against PLB (No.=110) in combination with MTX in RA patients with inadequate response to MTX alone. The primary endpoint was ACR20 response rate and DAS28 at 6 months.
At 6 months, in both treatment arms (ABA and IFB), about twice as many patients reached a DAS28≤3.2 (ABA 20.7% vs IFB 25.6%) compared to MTX+PLB (10.8%) and more than three achieved remission (DAS28≤2.6) (ABA 11.3% vs IFB 12.8%) compared to MTX (2.9%). Interestingly, at one year the ABA group data tended to increase (low activity 35.3% and remission 18.7%) compared to the stagnation of the results obtained with IFB (22.4% and 12.2%, respectively) with a mean reduction in DAS28 values at 6 and 12 months to −2.55 and −2.88units in the ABA group and −2.25 vs −2.25units in the IFB group (vs −1.48 in the placebo group, P<.001). ACR data at all times were similar in both treatment groups and superior to MTX, even from the 6th month (ACR50 and 70: ABA: 40.4% and 20.5%, IFB: 37% and 24.2%, MTX: 20% and 9.1%, P<.05), but at one year they were increased significantly in the group treated with ABA (ABA: 45.5% and 26.3%) while stagnated in the case of IFB (36.4% and 20.6%).8
Despite the limitations in this study on the systematic use of IFB only 3mg/kg, the effectiveness of ABA was clear, behaving similarly to IFB in the first months, with a trend in to overcome it in efficacy in the follow-up.
The 2-year follow-up data were presented in EULAR 2009 in Copenhagen, which included more than 90% of patients initially treated, showing an increase in the low-activity (42% at 2nd year for 35.3% in the first) and remission data (26% vs 18.7%) in the ABA+MTX group. These results were similar in the group treated with MTX+IFB who after completing a year changed to ABA+MTX, increasing the rate of responders in both low activity (45% vs 22.4%) and remission (29% vs 12.2%), as in the ACR of 2nd compared to the 1st year (ACR50 71% vs 36.4%, ACR70 45% vs 20.6%).9,10
Phase IIb TrialThis was a multicenter, randomized, placebo-controlled study to evaluate the safety and clinical efficacy at 12 months of two different doses of ABA: 2mg/kg (No.=105) and 10mg/kg (No.=115) or placebo (No.=119), in combination with MTX and administered intravenously in patients with active RA with inadequate response to MTX.11 At one year follow up, patients were switched to a fixed dose of 10mg/kg/4 week, as the dose of 2mg/kg/4 week showed no significant differences to PLB, with an open-label 5 year12 and 7 year13 extension.
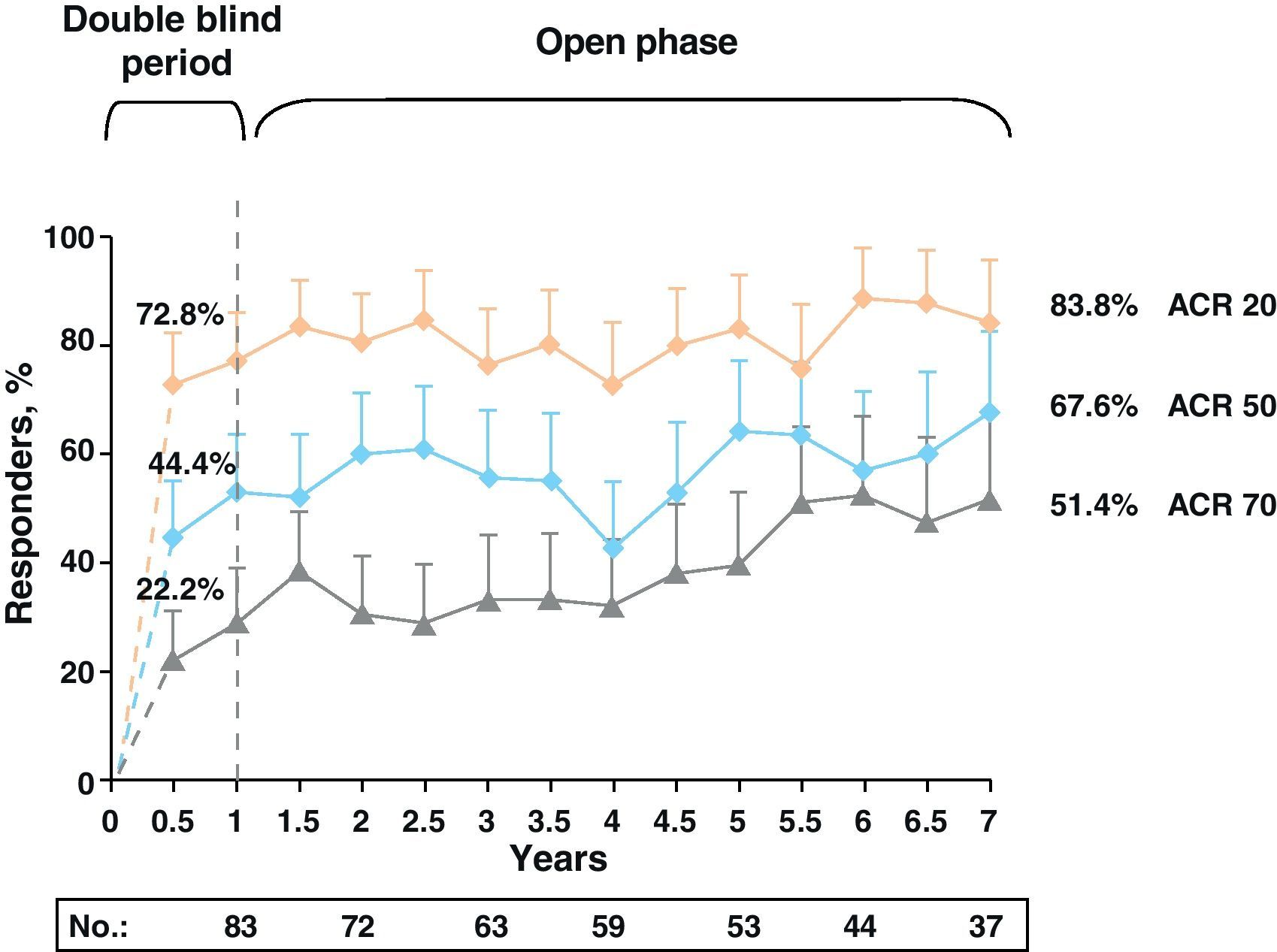
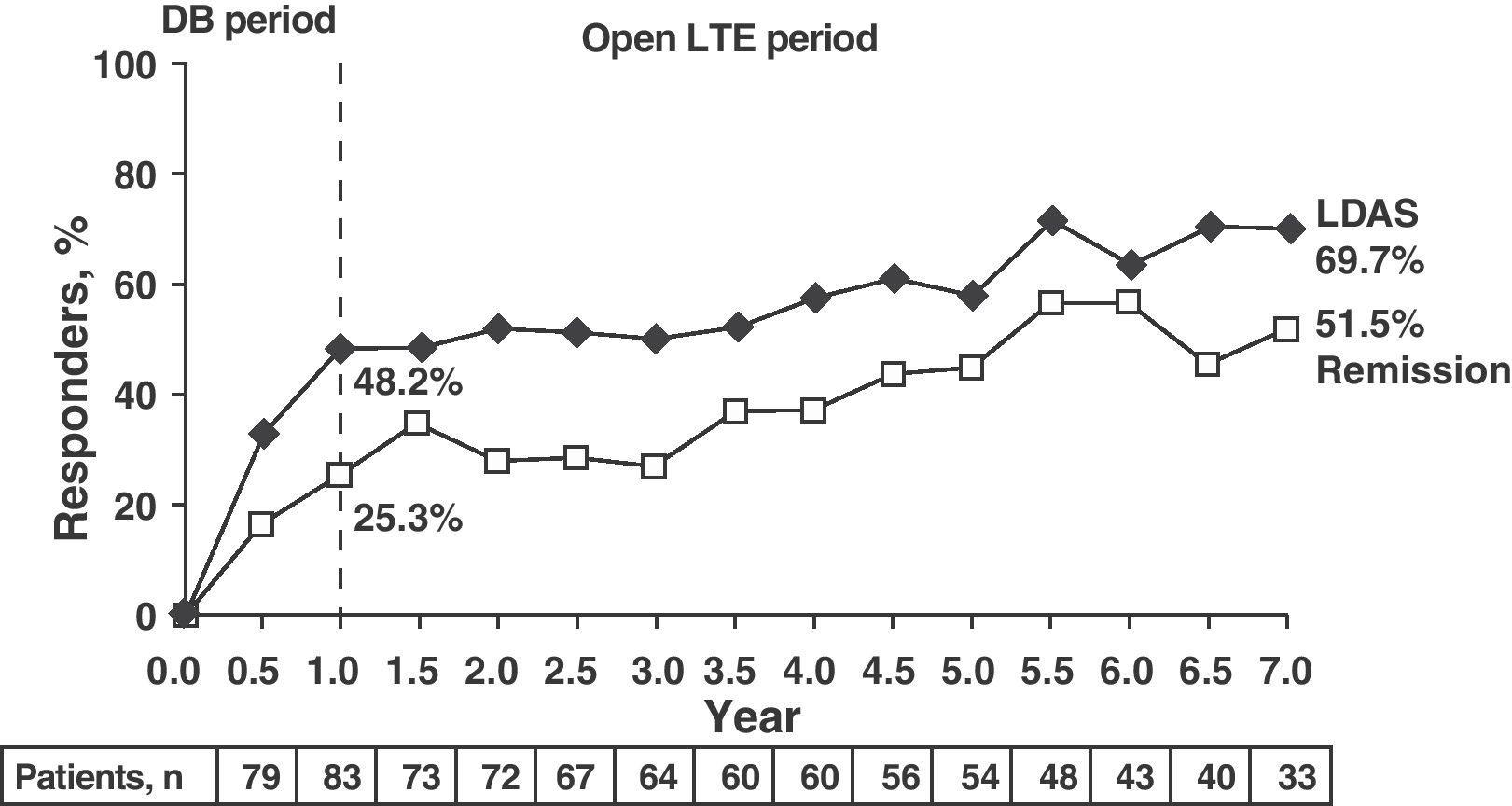
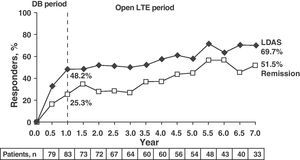
Clinical response data increased gradually over time (DAS28≤3.2: 48.2%, 58.5%, and 69.7% and DAS28≤2.6: 25.3%, 45.3%, and 51.5% for 1, 5, and 7 years, respectively). The same occurred with the ACR70 response rates, which were significant from 1st to the 7th year (28.9% vs 51.4%).
Note the high rates of remission (DAS28≤2.6) and ACR70 responses achieved at seven years in more than half of patients (51.5% and 51.4%) (Fig. 4).13
LDAS and DAS28 remission at 7 years in patients treated with abatacept. Data are based on all of the patients originally randomized to abatacept 10mg/kg who entered LTE, with visit data (analysis “observed cases”); remission defined as DAS28 (PCR)=DAS28<2.6; LDAS=DAS28 (CRP)≤3.2. DAS28: disease activity score; DB: double blind; LTE: long term extension; CI: confidence interval; LDAS: low disease activity score.
This study was performed in patients with RA, who were MTX naïve, had <2 years of disease progression and poor prognostic factors (≥12 tender joints, ≥10 swollen joints, CRP≥0.45mg/dl, rheumatoid factor and/or anti-CCP positive and radiographic evidence of erosion in the hands, wrists or feet). In this study, patients were randomized 1:1 to receive 10mg/kg/4 weeks ABA+MTX or PLB+MTX.
At one year, the treatment group (ABA+MTX) reached ACR50 57.4% vs 42.3% in the MTX+PLB, and a DAS28 remission rate≤2.6 from 41.4% vs 23.3%, achieving global reductions in DAS28 of −3.22 (vs −2.49) (P<.001).14
In this study, progression of structural damage data using radiographic follow up was obtained. The data were similar to those obtained in the AIM study, and, at one year, the ABA+MTX group had a mean change in total score of 0.63 vs 1.06 in the MTX group+PLB using a Genant modified TSS. It was also noted that patients in ABA+MTX group had a change greater than or equal to 0.3units compared to the baseline data in the HAQ-DI and a significant improvement in the physical and mental component SF-36.15
Results of Clinical SafetySafety studies have always occupied a very important role in the development of biological drugs in rheumatology. In the AIM, ATTEST, AGREE, and Phase IIb trial, ABA safety data were also widely reported. Table 1 summarizes the data on infections, symptoms and autoimmune diseases, malignancies and deaths observed in these studies and in periods of extension.
ABA: Abatacept; IFB: Infliximab; MTX: Methotrexate; Pat: Patients; PLB: Placebo.
| Groups | Infections | Severe Infections | Autoimmune Symptoms and Diseases | Neoplasia | Deaths |
| AIM | |||||
| PLB+MTXNo.=2191 year | 5 pat (2.3%) | 2 pat (0.9%) | 10 pat (4.6%) | 2 pat (0.9%) | 1 pat (0.2%) |
| ABA+MTXNo.=4331 year | 17 pat (3.9%); 90.5/100 pat-years | 11 pat (2.5%); 4.2/100 pat-years | 20 pat (4.6%) | 4 pat (0.9%) | 1 pat (0.2%) |
| ABA+MTXNo.=5935 years | a67.1/100 pat-years | 2.8/100 pat-years | 49 pat (8.2%) | b33 pat (5.6%) | 3 pat (0.5%) |
| ATTEST | |||||
| IFB+MTXNo.=1651 year | 14 pat (8.5%) | 1 pat (0.6%) | 2 pat (1.2%) | 2 pat (1.2%) | |
| ABA+MTXNo.=1561 year | c3 pat (1.9%) | 2 pat (1.3%) | 1 pat (0.6%) | 1 pat (0.6%) | |
| IIb | |||||
| ABA+MTXNo.=1201 year | 94.2/100 pat-years | 2.1/100 pat-years | 2.1/100 pat-years | 1 pat (0.5%) | |
| ABA+MTXNo.=1305 years | d77.3/100 pat-years | 3/100 pat-years | 12 pat (9.2%) | 1.5/100 pat-years | 5/100 pat-years |
| ABA+MTXNo.=1147 years | 3.18/100 pat-years | e13 pat (4.5%) | 20 pat (7%) | ||
| AGREE | |||||
| PLB+MTXNo.=2561 year | 139 pat (54.9%) | 5 pat (2%) | 5 pat (2%) | 0 pat (0%) | 4 pat (1.6%) |
| ABA+MTXNo.=2531 year | 132 pat (51.6%) | 5 pat (2%) | 6 pat (2.3%) | f1 pat (0.4%) | 2 pat (0.8%) |
The most common serious infections recorded during the first 2 years of the follow up period were pneumonia, acute bronchitis, cellulitis, and urinary4 tract infection.
Within tumor diagnoses reported during the 5 years of the extension period are non-melanocytic skin tumors, tumors of solid organ and hematologic malignancies.4
The most common serious infection was pneumonia (1.3 ABA vs IFB 1.8%) as well as 5 cases of serious opportunistic infections (including 2 cases of tuberculosis) in the IFB arm, while ABA was associated to none.16
The most common infections were pneumonia (1%) and diverticulitis (1%). There were no opportunistic infections or cases of tuberculosis.12
In the cumulative period of 7 years, a total of 13 patients developed autoimmunity or autoimmune disease. The most frequent was psoriasis, but cutaneous vasculitis, rheumatoid vasculitis, erythemanodosum, sicca syndrome, and multiple sclerosis12 were also seen.
In this study, ABA was well tolerated, with more frequent mild adverse effects (AE) (headache, nasopharyngitis and nausea).10 The overall incidence of AE remained stable at 5 years (cumulative incidence rate of 300.2 and 242.3 per 100 patient-years).4
Severe adverse effects (SAE) (17.7/100 patient-years at 12 months and 13.9/100 patient-years to 5 years) were more frequent in the ABA+MTX group (15%) than in the PLB+MTX (11.9%). The most common, excluding outbreaks of arthritis, were pneumonia, basal cell carcinoma and chest pain that occurred in more than 0.5% of patients during the 5-year extension period.
ATTEST StudyAs already mentioned, the ATTEST trial is interesting because it assesses the safety profiles of biological agents with 2 different mechanisms of action (ABA and IFB) under the same conditions of study. During the 12-month double-blind period, the AE related to the administration of treatment (infusions) and discontinuations due to SAE were lower in the ABA treated group than in the IFB treated one (total AE 89.1% vs 93.3%, treatment-related AE 46.2% vs 58.2%, and discontinuations due to SAE 3.2% vs 7.3%). The same applies to the SAE, which were up to 2 times more frequent in the IFB group (total SAE: 9.6% vs 18.2%, treatment-related SAE 3.2% vs 8.5%; SAE treatment interruptions 2.6% vs 3.6%).8 This frequency was maintained during the second year of follow-up of these patients, and in the extension period (AE: 15.76 in the double blind phase vs 9.96/100 patient-years in the extension period).9 In addition, there were 3 times less acute infusion reactions in the ABA group than in the IFB group (7.1% vs 24.8%). The most common infusion reactions were hypotension and urticaria that occurred only in the group treated with IFB (0% vs 4.8%), followed by headache and nausea (1.3% vs 4.2% and 1.9% vs 4.2%, respectively).8
Phase IIb TrialAccording to data from this study, ABA was well tolerated and the safety profiles of both doses used (2mg/kg and 10mg/kg) were similar to those in the placebo group.
The incidence of AE and SAE remained similar after 7 years of follow up as compared to the double-blind period (AE 366.1 and SAE 17.4/100 patient-years vs AE 489.7 and SAE 20/100 patient-years).13 The most frequent adverse effects were: nasopharyngitis (30.3%), respiratory infections (23%), cough (22.3%), headache (23.7%), nausea (15.3%) and diarrhea (19.9%).12
AGREE StudyDuring follow up, the frequency of AE, SAE, and SAE treatment discontinuations was similar in both groups (AE: 84.8% vs 83.4%; SAE: 7.8% vs 7.9%; SAE discontinuations: 1.2% vs 1.2%). The most frequent AE were mild nausea, upper respiratory tract infection and headache. Up to 16 patients (6.3%) experienced an acute infusional reaction in the treatment group, whereas in the MTX group there were only 5 (2%), all mild or moderate, except for a severe case of urticaria that presented in the group treated with ABA.
Two patients in the group treated with ABA became pregnant during the study. One patient who had received a dose of ABA submitted a positive urine pregnancy test on day 1 and suffered a spontaneous abortion between days 1 and 30. The other patient had received 9 infusions of the drug when she had a positive urine pregnancy test (day 253), confirmed by ultrasound, and then proceeded to an induced abortion at 281 days. Both patients were withdrawn from the study.14
ConclusionsFrom the efficacy and safety of these studies data we concluded that ABA associated with methotrexate is an effective treatment option with a good safety profile for patients with rheumatoid arthritis, including those with an aggressive onset, or those who have not been previously treated with other biologic therapies.
Conflict of InterestDr. AEC has participated as an advisor to BMS, lecturing in national forums on efficacy and safety of abatacept. Dr. FDG has participated as an advisor to BMS in national and international forums and has lectured on efficacy and safety of abatacept and has received funding for clinical and basic research from MSD, Pfizer, Roche and UCB. The remaining authors declare no conflict of interest.
Please cite this article as: Escudero Contreras A, et al. Eficacia y seguridad de abatacept en pacientes con artritis reumatoide sin tratamiento biológico previo. Reumatol Clin. 2011;7:392–6.