To assess the efficacy and safety of golimumab in the 140 patients included in Spain as the first part of the GO-MORE trial, a multinational study involving patients with active rheumatoid arthritis (RA) despite treatment with different disease-modifying antirheumatic drugs (DMARDs).
Patients and methodsThe patients received subcutaneous golimumab 50mg once a month during 6 months. The primary endpoint was the percentage of individuals with a good or moderate EULAR DAS28-ESR response after 6 months of treatment.
ResultsA total of 140 patients were included. Of these, 76.4% had very active disease (DAS28-ESR >5.1). 76.4% were taking methotrexate, 40.0% other DMARDs in monotherapy or combined, and 65.0% received corticosteroids. After 6 months, 82.9% of the patients showed a good or moderate EULAR response, 41.4% had low disease activity, and 30.7% were in remission. The percentage of responders one month after the first dose was 69.3%. The efficacy was similar in patients treated with methotrexate or other DMARDs, with different methotrexate doses, with or without corticosteroids, or in subjects who had failed one or more DMARDs. The response to golimumab was observed from the first dose. Golimumab was well tolerated and its safety profile was consistent with the findings of previous studies. Serious adverse events were reported in 11 patients (7.9%).
ConclusionThe addition of subcutaneous golimumab 50mg once a month to different DMARDs in patients with active RA yielded a moderate or good response after 6 months in 82.9% of the cases. The response was observed early, from the start of the second month, after a single dose of golimumab.
Analizar la eficacia y la seguridad de golimumab en los 140 pacientes incluidos en España en la parte 1 del estudio GO-MORE, un estudio multinacional en artritis reumatoide (AR) activa a pesar del tratamiento con distintos fármacos antirreumáticos modificadores de la enfermedad (FAME).
Pacientes y métodosLos pacientes recibieron golimumab 50mg subcutáneo una vez al mes durante 6 meses. El criterio de valoración principal fue el porcentaje con respuesta DAS28-VSG EULAR buena o moderada tras 6 meses de tratamiento.
ResultadosSe incluyó a 140 pacientes. El 76,4% tenía enfermedad muy activa (DAS28-VSG >5,1). El 76,4% estaba tomando metotrexato, el 40,0% otros FAME en monoterapia o combinación, y el 65,0% esteroides. Al mes 6, el 82,9% de los pacientes logró una respuesta EULAR buena o moderada, el 41,4% alcanzó baja actividad y el 30,7% remisión. El porcentaje de pacientes con respuesta al mes de la primera dosis administrada fue del 69,3%. La eficacia fue similar en pacientes tratados con metotrexato u otro FAME, distintas dosis de metotrexato, con/sin esteroides o que habían fallado a uno o más FAME. El golimumab fue bien tolerado y el perfil de seguridad fue coherente con estudios previos. Se comunicaron acontecimientos adversos graves en 11 pacientes (7,9%).
ConclusiónLa adición de golimumab 50mg subcutáneo mensual a distintos FAME en pacientes con AR activa deparó una respuesta moderada o buena a los 6 meses en el 82,9%. La respuesta comenzó a observarse tempranamente, ya al inicio del mes 2, tras una única dosis de golimumab.
The goal of treatment of rheumatoid arthritis (RA) is to achieve a state of clinical remission or, at least, low disease activity1–3 and prevent the progression of joint damage. The role of TNF-alpha antagonist drugs in this regard is well established in those patients in whom treatment with disease modifying antirheumatic drugs (DMARDs), including methotrexate (MTX),2,4 has previously failed. Nevertheless, most clinical trials with anti-TNF-alpha have been conducted in patients who failed to MTX and in combination with the latter, and experience in combinations with other DMARDs is limited.
Since, in clinical practice, a percentage of patients do not use MTX as a DMARDF, or uses it at doses other than those required in clinical trials, it would be interesting to obtain information on the effectiveness of anti-TNF-alpha in patient populations more similar to clinical practice, and assess patients with measurements outcomes closer to those normally used. In Spain, most patients used MTX, although around 40% may be treated with other DMARDs with or without concomitant MTX.5,6
Golimumab is a monthly subcutaneous anti-TNF-alpha monoclonal antibody that has shown clinical efficacy in a broad range of patients with RA (naïve to MTX, or after MTX failure, after failure of at least one anti-TNF-alpha7–9) and inhibits the progression of joint damage in conventional radiology.10 The GO-MORE study evaluated the efficacy and safety of golimumab in patients with active RA despite treatment with different DMARDs in 3280 patients from 40 countries with a profile similar to11 patients from clinical practice. The study had two parts: Part 1, the efficacy of subcutaneous golimumab assessed in clinical practice, and in Part 2 it assessed whether a combination of intravenous and subcutaneous golimumab could improve the initial response to subcutaneous golimumab.11 The main results of Part 1 showed that after 6 months of treatment, golimumab 50mg was effective and well tolerated: 82% of patients achieved a good or moderate response according to the European League Against Rheumatism (EULAR) criteria and 24% achieved clinical remission. This study involved countries from different geographical areas. In these cases, it is possible that different standards of treatment may influence certain outcomes that differs by region, so we think the analysis of a specific country would be interesting when the sample size allows it. Therefore, this paper presents the results of efficacy and safety of golimumab 50mg subcutaneous administered once a month in 140 patients in Spain from Part 1 of the study.
Patients and MethodsThe GO-MORE trial was an open, multicenter, international, prospective study (Protocol P06129; NCT00975130) in patients with active RA despite treatment with DMARDs and who had not previously been treated with biological drugs.11 The study was approved by the Ethics Committees of the participating hospitals and conducted according to the rules of Good Clinical Practice and the Declaration of Helsinki.
Part 1 of the study included patients 18 years of age or over, diagnosed with RA (according to the revised criteria of the American College of Rheumatology 1987) and active disease (DAS28-ESR ≥3.2) despite treatment with at least one of the following DMARDs at stable doses for at least one month: MTX, sulfasalazine, chloroquine, hydroxychloroquine, leflunomide, gold salts, azathioprine, or cyclosporine. Patients with active tuberculosis, untreated latent tuberculosis, moderate to severe heart failure or any other contraindication to treatment with anti-TNF-alpha were excluded.
Candidate patients who agreed to participate in the study were treated with subcutaneous golimumab 50mg once a month for 6 months. DMARD dose remained stable throughout the study. The primary endpoint of Part 1 was the percentage of patients with good or moderate EULAR response on the scale after 6 months of treatment, (defined as improvement in DAS28-ESR >1.2 from baseline or a score of 0.6–1.2 in those with baseline score ≤5.1). Evaluations were performed at the beginning of month 2 (after a dose of golimumab), beginning of month 4 (after 3 doses) and at the end of month 6 (after 6 doses of golimumab). Other secondary endpoints included the percentage of patients with low activity (DAS28-ESR <3.2), the percentage who achieved clinical remission (DAS28-ESR <2.6) and efficacy according to the Simplified Disease Activity Index (SDAI). The percentage of patients achieving little or no disability (Health Assessment Questionnaire Disability Index [HAQ-DI] ≤0.5) was also assessed, as was the quality of life using the European Quality of Life 5 Dimensions questionnaire (EQ-5D) and the percentage of patients considered the symptoms as acceptable, based on the question: “Considering how the disease affects you in your different activities, if continuing in this state in the coming months, would you consider it satisfactory?”. In this first part, involving 3280 patients in 40 countries, 140 patients were recruited in Spain.
In part 2 of the study, patients who had achieved a moderate or good DAS28-ESR EULAR response but had not achieved remission at the end of month 6 were included. These patients were randomized to continue golimumab 50mg subcutaneously once a month, or a combination of golimumab subcutaneous and intravenously for 6 months, in order to evaluate the effectiveness of both regimens in the percentage of patients reaching remission between month 11 and the end of month 12. In this second part, Spain included few patients, so no results are presented.
Statistical AnalysisThe sample size calculated for the first part of the study was 3150 patients, which allowed the detection of small differences in efficacy between the different subgroups.11 The analysis presented in the Spanish sample (n=140) is not enough to show differences between subgroups and, due to lack of power, is mostly descriptive. The patients evaluated for efficacy were given at least one dose of the drug and had at least one follow-up visit and patients lost or for whom an evaluation was not available were classified as nonresponders.
The EULAR response was assessed in the overall population and then stratified by different variables: DMARD used as background therapy, dose of MTX (low [<10mg/week], medium [≥10–<15mg/week], high [≥15mg/week]), concomitant glucocorticoid treatment (yes/no) and number of DMARD to which the patient had failed.
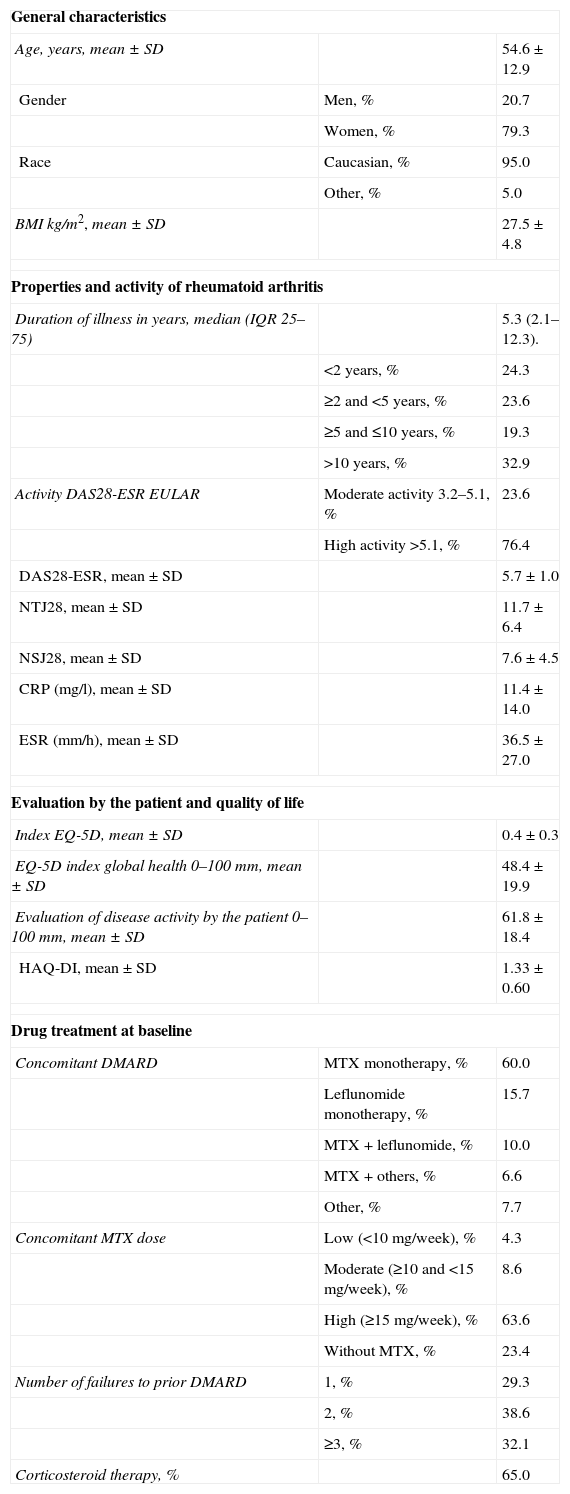
ResultsDescription of the SampleIn Spain, 140 patients, 111 women (79.3%) and 29 men (20.7%) were included. The median duration of disease was 5.3 years (IQR 2.1–12.3). 76.4% of patients had high activity (DAS28-ESR >5.1). 76.4% were taking MTX as a DMARD (60% as monotherapy and 16.4% in combination with another drug) and 40.0% were receiving a DMARD other than MTX (mainly leflunomide). Most patients were taking high doses of MTX (≥15mg/week) and 65% were receiving glucocorticoids. 70.7% had experienced more than one DMARD failure. Baseline characteristics are described in Table 1. The characteristics of the Spanish sample were similar to those of the overall sample,11 except for a higher prevalence of Caucasian patients (95% in our sample, 70% in the overall sample) and a slightly higher percentage of use of MTX monotherapy (60% vs 51%). The disease characteristics (duration, activity at baseline, treatment with steroids, MTX dose or number of DMARD failures) were also similar to the overall sample.
Baseline Characteristics of the Subjects Included in the Study (n=140).
| General characteristics | ||
| Age, years, mean±SD | 54.6±12.9 | |
| Gender | Men, % | 20.7 |
| Women, % | 79.3 | |
| Race | Caucasian, % | 95.0 |
| Other, % | 5.0 | |
| BMI kg/m2, mean±SD | 27.5±4.8 | |
| Properties and activity of rheumatoid arthritis | ||
| Duration of illness in years, median (IQR 25–75) | 5.3 (2.1–12.3). | |
| <2 years, % | 24.3 | |
| ≥2 and <5 years, % | 23.6 | |
| ≥5 and ≤10 years, % | 19.3 | |
| >10 years, % | 32.9 | |
| Activity DAS28-ESR EULAR | Moderate activity 3.2–5.1, % | 23.6 |
| High activity >5.1, % | 76.4 | |
| DAS28-ESR, mean±SD | 5.7±1.0 | |
| NTJ28, mean±SD | 11.7±6.4 | |
| NSJ28, mean±SD | 7.6±4.5 | |
| CRP (mg/l), mean±SD | 11.4±14.0 | |
| ESR (mm/h), mean±SD | 36.5±27.0 | |
| Evaluation by the patient and quality of life | ||
| Index EQ-5D, mean±SD | 0.4±0.3 | |
| EQ-5D index global health0–100mm, mean±SD | 48.4±19.9 | |
| Evaluation of disease activity by the patient 0–100mm, mean±SD | 61.8±18.4 | |
| HAQ-DI, mean±SD | 1.33±0.60 | |
| Drug treatment at baseline | ||
| Concomitant DMARD | MTX monotherapy, % | 60.0 |
| Leflunomide monotherapy, % | 15.7 | |
| MTX+leflunomide, % | 10.0 | |
| MTX+others, % | 6.6 | |
| Other, % | 7.7 | |
| Concomitant MTX dose | Low (<10mg/week), % | 4.3 |
| Moderate (≥10 and <15mg/week), % | 8.6 | |
| High (≥15mg/week), % | 63.6 | |
| Without MTX, % | 23.4 | |
| Number of failures to prior DMARD | 1, % | 29.3 |
| 2, % | 38.6 | |
| ≥3, % | 32.1 | |
| Corticosteroid therapy, % | 65.0 | |
DAS28: 28-Joint Disease Activity Score; SD: standard deviation; EQ-5D: European Quality of Life 5 Dimensions; EULAR: European League Against Rheumatism; DMARD: disease-modifying antirheumatic drug; HAQ-DI: Health Assessment Questionnaire Disability Index; MTX: methotrexate; NTJ: number of painful joints; NSJ: number of swollen joints; CRP: C-reactive protein; IQR: interquartile range; ESR: erythrocyte sedimentation rate.
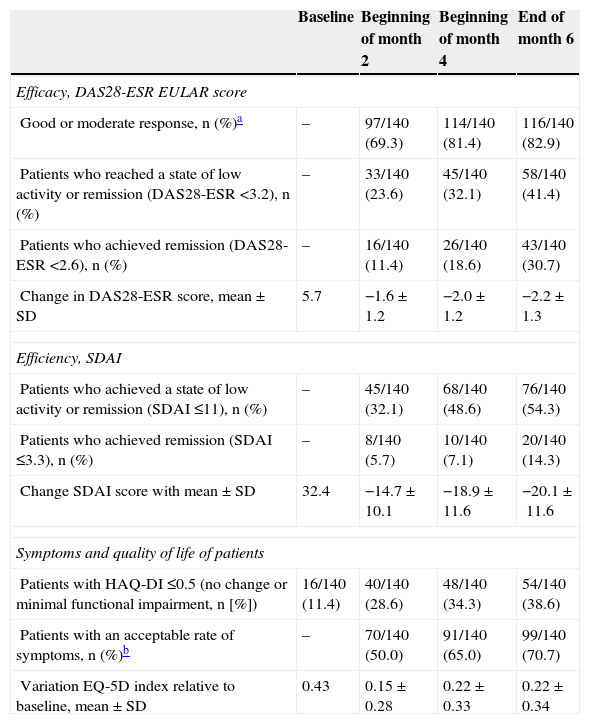
A good or moderate EULAR response was seen in 82.9% of patients (116 of the 140 patients in the ITT population who received at least one dose of study medication, 95% confidence interval, 75.8–88.2). The response was quick. 69.3% of patients had achieved response to treatment a month after a single dose of golimumab; the response increased to 81.4% at the start of month 4 (after 3 doses of golimumab) and remained similar until the evaluation of the end of month 6.
At the end of six months, 41.4% of patients had achieved a state of low disease activity (DAS28-ESR <3.2) and 30.7% had achieved remission (DAS28-ESR <2.6). The percentage of patients achieving these objectives in every evaluation is described in Table 2. Unlike clinical response, which at the beginning of month 4 was already close to the maximum, the percentage who achieved remission or low activity increased with time. The activity measured by the SDAI index also declined, and by the sixth month the percentage who achieved remission or low activity was 54.5%, while 14.3% achieved remission (Table 2).
Percentage of Responders and Evolution of the Parameters of Activity.
| Baseline | Beginning of month 2 | Beginning of month 4 | End of month 6 | |
|---|---|---|---|---|
| Efficacy, DAS28-ESR EULAR score | ||||
| Good or moderate response, n (%)a | – | 97/140 (69.3) | 114/140 (81.4) | 116/140 (82.9) |
| Patients who reached a state of low activity or remission (DAS28-ESR <3.2), n (%) | – | 33/140 (23.6) | 45/140 (32.1) | 58/140 (41.4) |
| Patients who achieved remission (DAS28-ESR <2.6), n (%) | – | 16/140 (11.4) | 26/140 (18.6) | 43/140 (30.7) |
| Change in DAS28-ESR score, mean±SD | 5.7 | −1.6±1.2 | −2.0±1.2 | −2.2±1.3 |
| Efficiency, SDAI | ||||
| Patients who achieved a state of low activity or remission (SDAI ≤11), n (%) | – | 45/140 (32.1) | 68/140 (48.6) | 76/140 (54.3) |
| Patients who achieved remission (SDAI ≤3.3), n (%) | – | 8/140 (5.7) | 10/140 (7.1) | 20/140 (14.3) |
| Change SDAI score with mean±SD | 32.4 | −14.7±10.1 | −18.9±11.6 | −20.1±11.6 |
| Symptoms and quality of life of patients | ||||
| Patients with HAQ-DI ≤0.5 (no change or minimal functional impairment, n [%]) | 16/140 (11.4) | 40/140 (28.6) | 48/140 (34.3) | 54/140 (38.6) |
| Patients with an acceptable rate of symptoms, n (%)b | – | 70/140 (50.0) | 91/140 (65.0) | 99/140 (70.7) |
| Variation EQ-5D index relative to baseline, mean±SD | 0.43 | 0.15±0.28 | 0.22±0.33 | 0.22±0.34 |
DAS28: 28-Joint Disease Activity Score; EQ-5D: European Quality of Life 5 Dimensions; EULAR: European League Against Rheumatism; HAQ-DI: Health Assessment Questionnaire Disability Index; SDAI: Simplified Disease Activity Index; ESR: erythrocyte sedimentation rate.
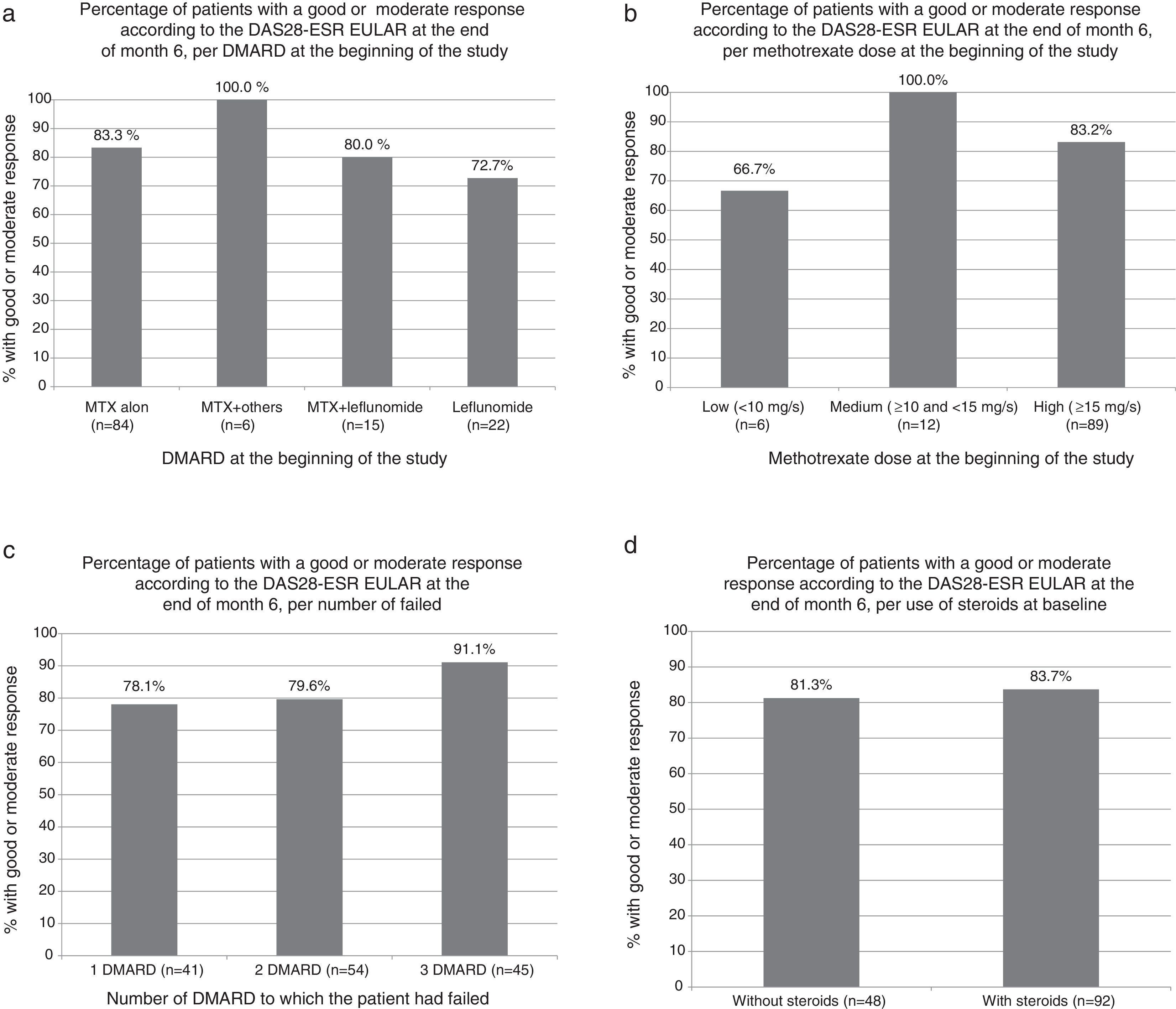
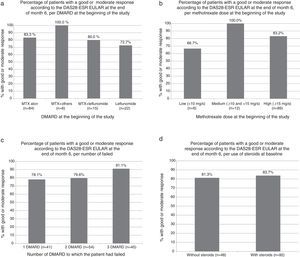
The percentage of patients achieving good or moderate EULAR response was similar in patients who were treated with MTX, with other DMARDs or combination of MTX and other DMARDs. No differences were observed in the response depending on the dose of MTX, DMARD number to which the patient had previously failed, or the treatment (or lack thereof) with steroids (Fig. 1a–d).
Percentage of patients with good or moderate response according to DAS28-ESR EULAR score at the end of month 6. (a) According to DMARD at baseline (P=NS for all comparisons relative to the methotrexate alone group, Fisher's exact test). (b) Depending on the dose of methotrexate at baseline (P=NS for all comparisons, Fisher exact test). (c) Depending on the number of DMARD to which the patient had failed (P=NS for all comparisons, Fisher exact test). (d) According to the use of steroids at baseline visit (P=NS, Fisher exact test). DMARD, disease modifying antirheumatic drug.
Table 2 shows the evolution of symptoms and quality of life as reported by patients. Quality of life, functional impairment and symptomatic status of patients improved over time in parallel with the improvement in DAS28-ESR. The percentage of patients that considered their symptoms as acceptable after 6 months of treatment was 70.7% and the percentage with HAQ-DI ≤0.5 (status unchanged or minimal functional impairment) was 38.6%. We also observed an improvement in quality of life measured by the EQ-5D (Table 2) index. The improvement of these indices was noted at the evaluation at one month of receiving the first dose of golimumab.
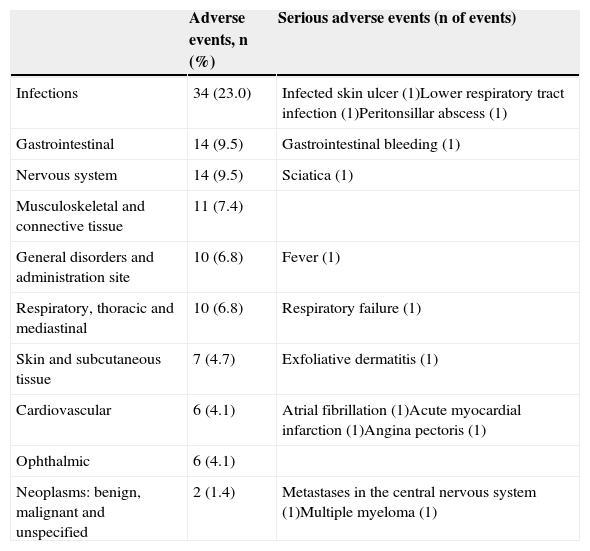
Tolerability and SafetyGolimumab was well tolerated. Reactions at the injection site were seen in only 2 patients (1.4%) and were mild and did not cause discontinuation of medication. The safety profile was consistent with previous studies of golimumab. Adverse events were observed in 50.0% of patients and in 34 patients (23.0%) were considered as potentially related to the drug. In 11 patients (7.9%), adverse events reported as severe (Table 3) were observed. The medication had to be discontinued in 6 patients (4.1%) due to adverse reactions. Elevated transaminases were observed in one patient (0.7%). Two deaths were reported, one due to multiple myeloma with acute renal failure (the patient had received 3 doses of golimumab and death occurred 48 days after the third dose) and another due to respiratory failure in the context of a respiratory infection where no specific germ was cultivated and atrial fibrillation (also after 3 doses and 71 days after the third dose). These deaths were considered by the investigators as possibly and probably related to the drug, respectively.
Number and Percentage of Patients With Adverse Events During the 6-Month Study.
| Adverse events, n (%) | Serious adverse events (n of events) | |
|---|---|---|
| Infections | 34 (23.0) | Infected skin ulcer (1)Lower respiratory tract infection (1)Peritonsillar abscess (1) |
| Gastrointestinal | 14 (9.5) | Gastrointestinal bleeding (1) |
| Nervous system | 14 (9.5) | Sciatica (1) |
| Musculoskeletal and connective tissue | 11 (7.4) | |
| General disorders and administration site | 10 (6.8) | Fever (1) |
| Respiratory, thoracic and mediastinal | 10 (6.8) | Respiratory failure (1) |
| Skin and subcutaneous tissue | 7 (4.7) | Exfoliative dermatitis (1) |
| Cardiovascular | 6 (4.1) | Atrial fibrillation (1)Acute myocardial infarction (1)Angina pectoris (1) |
| Ophthalmic | 6 (4.1) | |
| Neoplasms: benign, malignant and unspecified | 2 (1.4) | Metastases in the central nervous system (1)Multiple myeloma (1) |
The analysis of patients enrolled in the GO-MORE study in Spain shows that treatment with golimumab 50mg subcutaneous once a month added to different DMARD led to a good or moderate EULAR response at the sixth month in most patients (82.9%), and that this response is observed early. One month after the first dose, 69% of the patients had achieved response. Most patients who responded had done so at the beginning of month 4, after 3 doses of the drug, where the response rate was 81.4%. The data are similar to that found in the overall sample (76.9% at the beginning of the month 4 and 82.1% at the end of the month 611). In the example of Spain, also around 40% reached a low activity state (DAS28-ESR <3.2) and almost one-third reached remission (DAS28-ESR <2.6) at six months, a percentage which increased from month 2 in successive assessments (Table 2). This aspect is interesting because it suggests that while the clinical response is usually achieved within 12–14 weeks of treatment12 specific therapeutic objectives such as low activity or remission may take longer to achieve. The percentage of patients achieving low activity (54.3%) on SDAI was higher than that with DAS-28, while the percentage in SDAI remission (14.3%) was lower. This discrepancy may be due to the inclusion of the assessment of the general condition by the physician in the SDAI index but not in the DAS-28, which may be more stringent than the assessment made by patients accustomed to the symptoms of the disease but who have perceived improvement with treatment, as usually happened in the present study.
In general, multinational studies such as this typically include a high percentage of patients from geographical areas outside Europe or North America. In the Spanish sample, the percentages of patients achieving low activity (41.4%) and remission (30.7%) were higher than those observed in countries in other geographic areas such as Asia and Latin America.13 This may be due to access to the fact that medication and treatment standards are different and patients can enter clinical trials in more advanced disease or more disease activity. When a quantitative change is measured (as is the clinical response in terms of change of DAS28-ESR), the success rates may be similar between countries, whereas when a specific objective to be achieved is evaluated as in DAS28-ESR <3.2 or DAS28-ESR <2.6, the quality of baseline treatment and standards of care received, and the status of the subjects that start when they enter the study, establish different success rates. Thus, the percentages of patients who achieved remission in GO-MORE countries of Asia and Latin America were less than 20%, in part, probably because patients in these regions entered the study with higher activity.13 Since these differences have an impact on the overall success rates of clinical trials, we believe it is important to perform specific analysis of each country or region, provided that the sample size allows it, and even with the limitations involved, and the analysis of data from Spain meets this objective.
In line with this, our data add value by also showing the efficacy of golimumab in routine clinical practice in which, although MTX is the most commonly used DMARD, other DMARDs are also used. In our sample, 40.0% were receiving a DMARD other than MTX (of which 16.4% was taking it associated with MTX and 23.6% in monotherapy). After MTX, the most commonly used DMARD was leflunomide. The percentage of patients with each DMARD was similar to that described in the EMAR II study where more MTX was the most used DMARD (59.6%) in the group of patients with RA,5 followed by leflunomide (22.1%), antimalarials (12.2%) and sulfasalazine (3.1%), and in addition, 20.7% of patients received 2 or more DMARDs simultaneously. In another study in which patients who received a first DMARD,6 the most frequently used drug was MTX monotherapy (81.3%), followed by leflunomide (4.1%) and hydroxychloroquine (3.2%). We believe, therefore, that the Spanish GO-MORE study is representative of Spanish RA clinical practice and therefore the results are applicable to a broad population of patients with RA, those in which DMARD (one or more) have failed and require anti-TNF-alpha. As additional findings, the efficacy of golimumab was similar in patients with different doses of MTX, with failure to one or more DMARDs or independent of steroid treatment, which strengthens the results ahead of their practical application.
Golimumab was well tolerated. The low incidence of reactions at the injection site was of note (1.4% in the first 6 months). In this analysis and in the overall sample,11 the profile of adverse events reported were consistent with those described for golimumab in the pivotal clinical trials and product data sheet.7–9,12 The incidence of individual serious adverse events was also similar to that found in the studies of golimumab and are similar to adalimumab.14 There were 2 deaths (one for multiple myeloma with acute renal failure and other respiratory failure in the context of a lower respiratory tract infection in which no germ was found and accompanied by atrial fibrillation) in our sample. In the overall sample included in the safety analysis (n=3357) only 10 deaths were reported (6 in Part 1 and 4 additional more than 30 days after the last dose) without any predominant cause, of which 5 cases were classified by the investigators as possibly related and 5 unrelated to the drug11.
The main limitation of the study is that it has a small number of patients (recruited in Spain) and therefore is has insufficient statistical power to find differences, if any, in the efficacy of golimumab among different groups analyzed. In the overall GO-MORE study sample (n=3280), no significant differences in efficacy between the groups analyzed11 was found, so it was not expected to exist in our patients. Keep in mind that the first part of the GO-MORE study presented in this paper was open and uncontrolled, so the study is subject to the biases inherent in uncontrolled open studies. However, this analysis is of interest, first, to show the effect of golimumab in a population of patients who have failed one or more DMARDs including MTX or other, different DMARD, representing the RA population that usually requires treatment with anti-TNF-alpha in routine clinical practice in Spain and second, to show some differences in the efficacy of golimumab to achieve certain objectives (low activity or remission) when the results of Spain were compared to the overall sample or results in other countries. We believe that if the sample size permits it, and assuming the limitations in interpreting the results, the analysis of patients in Spain in large clinical trials, especially if they approach clinical practice as the present study, are necessary to better define efficacy and safety of drugs in patients who ordinarily are seen in our consultations.
In conclusion, the results of the GO-MORE study in Spain showed that golimumab is effective as an add-on to other DMARDs with or without steroids, in RA patients who have failed one or more DMARDs, with the DAS28-ESR response at the sixth month being good or moderate in a very high percentage of patients (82.9%), and associated to low activity or remission in a significant percentage of patients (41.4% and 30.7%, respectively). The onset of action was rapid, the profile of tolerability good and safety was consistent with that described in other clinical trials and the product data sheet.
Ethical ResponsibilitiesProtection of persons and animalsThe authors declare that the performed procedures conformed to the ethical standards of the committee responsible for human experimentation and were in accordance with the World Medical Association Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the protocols of the workplace on the publication of patient data.
Right to privacy and informed consentThe authors state that patient data do not appear in this article.
Conflict of InterestThe following authors declare conflicts of interest: Mary J. Arteaga and Luis Cea-Calvo (full-time employees at Merck Sharp & Dohme of Spain); Carlos M. Gonzalez (advisor to Merck Sharp & Dohme); María L. García Vivar (Roche and Janssen advisory, educational activities for Abbie, Pfizer, UCB, Roche, Merck Sharp & Dohme and Bristol-Myers Squibb); Raimon Sanmartí (grant by Merck Sharp & Dohme). The remaining authors declare no conflicts of interest.
The GO-MORE study was funded by Schering-Plough (now Merck & Co., Inc., Whitehouse Station, New Jersey, USA). The study involved researchers from 40 countries. We appreciate the feedback of researchers and their work in the inclusion and monitoring of the study patients.
Please cite this article as: Alonso A, González CM, Ballina J, García Vivar ML, Gómez-Reino JJ, Marenco JL, et al. Eficacia y seguridad de golimumab añadido a fármacos antirreumáticos modificadores de la enfermedad en artritis reumatoide. Resultados del estudio GO-MORE en España. Reumatol Clin. 2015;11:144–150.