Rhupus in an infrequent disease in which an overlap between lupus eritematosus and rheumatoid arthritis exists. Joint manifestations are prominent and treatment with nonbiological DMARDs is not always satisfactory, so immunosuppressors and biological agents have been tried.
A prospective, open clinical study was done to evaluate efficacy and tolerability of rituximab in patients with rhupus. The main objective was a change in DAS28 at 6 months and secondary objectives were a change in MEX-SLEDAI at 6 months, change in DAS28 and MEX-SLEDAI during follow up, steroid requirements and detection of adverse events.
We included 9 women with a mean age of 43 years and disease duration of 10 years. A significant reduction in DAS28 was observed (from 5.73 at baseline to 3.02 at 6 months, P<.001). Improvement in DAS28 was maintained during follow up. At 6 months, 3 patients were in remission and 3 had low disease activity. MEX-SLEDAI diminished from 5 points at baseline to 1.22 at 6 months (P<.001). There was a negative correlation between clinical improvement and anti-CCP levels (r=−0.794, P=.011). Mean prednisone dose was reduced from 11.66mg/day at baseline to 0.55 and 1.11mg/day at 12 and 24 months. Treatment was well tolerated.
In this study rituximab was effective not only for joint affection but also for other manifestations of the disease. We consider that this biological agent can be a good therapeutic option for patients with rhupus.
El rhupus es una entidad poco común en la que se superponen datos de lupus eritematoso generalizado y artritis reumatoide, predominando con frecuencia las manifestaciones articulares. En muchos casos el tratamiento con fármacos modificadores de la enfermedad no biológicos es insatisfactorio, por lo que se ha intentado el uso de inmunosupresores y fármacos biológicos.
Se realizó un estudio prospectivo y abierto para evaluar la eficacia y la tolerabilidad de rituximab en pacientes con rhupus. El objetivo principal fue el cambio en el DAS28 a los 6 meses; fueron objetivos secundarios el cambio en MEX-SLEDAI a los 6 meses, el cambio en DAS28 y MEX-SLEDAI durante el seguimiento, el requerimiento de esteroides y el registro de eventos adversos.
Se incluyó a 9 pacientes, todas mujeres, con edad promedio de 43 años y tiempo de evolución de 10 años. Se observó un descenso en la puntuación basal de DAS28 de 5,73 a 3,02 a los 6 meses (p<0,001). La mejoría en el DAS28 se mantuvo durante el periodo de seguimiento. A los 6 meses, 3 pacientes presentaban remisión por DAS28 y 3 actividad baja. La calificación de MEX-SLEDAI disminuyó de 5 puntos a nivel basal a 1,22 a los 6 meses (p<0,001) y mantuvo esta mejoría. Se observó una correlación negativa entre la mejoría clínica y los niveles de anti-CCP (r=−0,794; p=0,011). La dosis de prednisona disminuyó de 11,66mg/día basal a 0,55 y 1,11mg/día a los 12 y 24 meses, respectivamente. En general, el tratamiento con rituximab fue bien tolerado durante el estudio.
En los pacientes de nuestro estudio, el tratamiento con rituximab mostró ser eficaz tanto en las manifestaciones articulares, con reducción significativa del DAS28, como en otras manifestaciones de lupus, con mejoría del MEX-SLEDAI. Consideramos que esta puede ser una buena opción terapéutica para pacientes con rhupus.
Rhupus is defined as the superposition of lupus erythematosus (SLE) and rheumatoid arthritis (RA). It is a rare entity, with fewer than 150 cases reported in the literature. An epidemiological study found that the prevalence is about 0.09%.1 Although some authors argue that rhupus represents a subset of SLE with predominant joint manifestations and characteristics, clinical and serological evidence support that this is a definite overlap syndrome.2
In most rhupus cases described, the clinical picture starts with a symmetrical and erosive polyarthritis with positive rheumatoid factor and/or citrulline antipeptide antibodies (anti-CCP) and can be classified as RA. It also features other clinical manifestations of SLE and its specific antibodies (anti-dsDNA and/or anti-Sm). In addition to joint disease, the most common manifestations of lupus are mucocutaneous involvement, hematological abnormalities and serositis; renal involvement or central nervous system is uncommon. Arthropathy is usually the predominant manifestation in these patients, with clinical inflammation, deformities and erosions characteristics in RA and even rheumatoid nodules.1,3–5
In many of the described cases there was no good response to disease-modifying nonbiological drugs (DMARDs) and treatment has been attempted with immunosuppressants (azathioprine, mycophenolate mofetil) and even with biologics (abatacept).2,6
Despite the undoubted efficacy of anti-TNF drugs in RA patients, previous experience in SLE has shown little efficacy and in some cases even a worsening of symptoms, making them of little use for their use in patients with rhupus.7–9
Rituximab is a monoclonal antibody directed against the CD20 molecule on B lymphocytes which has been extensively demonstrated to be effective in the treatment of RA.10,11 In addition, although recent reports of controlled trials have not yielded the expected results,12–14 there are many reports in the literature of successful treatment with rituximab in patients with SLE and different types of manifestations, mainly hematological, renal and even central nervous system.15–17 There are, at this time, no specific reports on the response in joint manifestations, although surely many of these patients presented improvement.
The objective of this study was to investigate the efficacy and tolerability of rituximab in a group of patients with rhupus.
Patients and MethodsStudy DesignWe performed a prospective, open study to evaluate the efficacy of rituximab in patients with rhupus. The primary endpoint was change in DAS28 at 6 months (improvement defined as a decrease of at least 0.618). Secondary endpoints were the change in the MEX-SLEDAI at 6 months follow up to the end of the evaluation, the change in DAS28 at the end of monitoring and assessment and steroid requirements, and the recording of adverse event during the study. We included for analysis only patients who had completed at least 24 months follow-up.
We recorded demographic data, clinical features of the disease and the classification criteria for SLE and RA, and data evolution and previous response to different treatments. All patients signed an informed consent for treatment.
TreatmentPatients received rituximab 1g by intravenous infusion for 4h, after premedication with hydroxyzine, paracetamol and dexamethasone 8mg intravenously on days 1 and 15 of the study, with subsequent cycles every 9–12 months, depending on the clinical activity evaluated by DAS28.18 Nonbiological DMARDs (methotrexate, leflunomide, azulfidine) and immunosuppressants (azathioprine, mycophenolate mofetil, cyclophosphamide) were suspended one month before the start of the study. Concurrent use of low doses of steroids (maximum 15mg of prednisone/day or its equivalent) and hydroxychloroquine, and nonsteroidal anti-inflammatory analgesics and adjuvant therapy (lowering agents, antihypertensives, etc.) was allowed.
Study SubjectsThe study population consisted of consecutive patients over 18 years, diagnosed with rhupus according the criteria proposed by Simon et al.,3,19: symmetrical erosive polyarthritis, signs and symptoms of SLE and anti-dsDNA antibodies and/or anti-Sm and joint disease with moderate to severe activity, defined as DAS28≥3.2.18 For patients of childbearing age, we used a reliable contraceptive. All patients underwent screening for tuberculosis and viral hepatitis prior to treatment. Exclusion criteria included the presence of pregnancy or lactation, history of hepatitis, need to use other immunosuppressive drugs or concomitant DMARDs (except antimalarials) or active infection at baseline.
EvaluationsPatients were evaluated on a quarterly basis throughout the follow up. At each visit patients were questioned on symptoms suggestive of activity; we performed a complete physical examination by a rheumatologist and determined the DAS28 and MEX-SLEDAI scores and also recorded the presence of concomitant medications and adverse events intentionally. We also carried out routine laboratory studies that included CBC with differential and platelet count, blood chemistry, urinalysis, creatinine clearance, 24h urine albumin, acute phase reactants (erythrocyte sedimentation rate, C-reactive protein) and C3 and C4 levels.
Statistical AnalysisWe used descriptive statistics, Fisher's exact test for qualitative variables and Student's t test for quantitative variables. The comparison of differences was performed using the Wilcoxon rank test. We used SPSS version 15 in Spanish.
ResultsCharacteristics of the study subjects: nine patients were included, all women, with a mean age of 43 years (range 38–57 years) and a duration of illness of 10.03 years (range 18–22 years). In 6 patients the initial diagnosis was RA and preceded SLE manifestations by 2.5 years on average; in only 3 patients the initial diagnosis was SLE. Table 1 shows the demographic, clinical and serological features, and pretreatment of patients included and the criteria by which the diagnosis of rhupus was made. It should be mentioned that in all patients treated we previously employed NSAIDs, steroids in low and medium doses or non-biological DMARD monotherapy or combination therapy without success.
Clinical Characteristics of Patients and Serological Features.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total | |
| Age, years | 43 | 38 | 39 | 52 | 43 | 57 | 36 | 42 | 38 | 43 (38–57) |
| Sex, F/M | F | F | F | F | F | F | F | F | F | 9 (100%) |
| Years diagnosis | 18 | 22 | 12 | 5 | 10 | 13 | 6 | 1.8 | 2.5 | 10.03 (1.8–22) |
| Arthritis | + | + | + | + | + | + | + | + | + | 9 (100%) |
| Photosensitivity | + | + | + | + | + | + | 6 (66.6%) | |||
| Malar erythema | + | + | + | + | + | + | + | + | 8 (88.8%) | |
| Oral ulcers | + | + | + | + | + | + | + | 7 (77.7%) | ||
| Discoid lupus | + | 1 (11.1%) | ||||||||
| Serositis | + | 1 (11.1%) | ||||||||
| Renal | iii | ee | v | ii | 4 (44.4%) | |||||
| Neurological | 0 | |||||||||
| Hematological | LL | LL | LLT | LL | LL | LL | 6 (66.6%) | |||
| ESR/CRP + | + | + | + | + | + | + | + | + | + | 9 (100%) |
| FR | 72 | 410 | 752 | 681 | – | 212 | 74 | 4560 | 120 | 8 (88.8%) |
| Anti-CCP | 172 | 197 | 6 | 433 | 1260 | 311 | – | 300 | 224 | 8 (88.8%) |
| AAN | HD | Mg | HD | HD | HD | HD | HD | HD | LP | 9 (100%) |
| Anti-dsDNA | + | + | + | + | + | 5 (55.5%) | ||||
| Erosions Rx | + | + | + | + | + | + | + | + | 8 (88.8%) | |
| Criteria | ||||||||||
| RA | + | + | + | + | + | + | + | + | + | 9 (100%) |
| SLE | + | + | + | + | + | + | + | + | + | 9 (100%) |
| Pretreatment | ||||||||||
| Steroids | + | + | + | + | + | + | + | + | + | 9 (100%) |
| Antimalarial | + | + | + | + | + | + | + | 7 (77.7%) | ||
| Methotrexate | + | + | + | + | + | + | + | + | 8 (88.8%) | |
| Sulphasalazine | + | + | + | + | + | + | + | 7 (77.7%) | ||
| Azathioprine | + | + | + | 3 (33.3%) | ||||||
| Cyclophosphamide | + | 1 (11.1%) | ||||||||
| Mycophenolate | + | + | 2 (22.2%) | |||||||
ANA, antinuclear antibodies; anti-dsDNA, anti-dsDNA; anti-CCP, anti-citrullinated peptide antibodies; RA, rheumatoid arthritis; F, female; FR, rheumatoid factor; HD, homogeneous diffuse; SLE, systemic lupus erythematosus; LL, leukolymphopenia; LLT, leukolymphopenia and thrombocytopenia; LP, peripheral; M, male; MG, spotted.
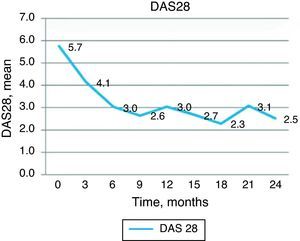
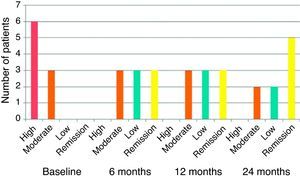
Clinical efficacy: good clinical response was observed, with decrease in the baseline DAS28 score from 5.73 to 4.13 at 3 months and 3.02 at 6 months, a statistically significant change (P<.001). Improvement in DAS28 was maintained during the follow up period, with a DAS 28 score of 3.04 at 12 months and 2.52 at 24 months, as shown in Fig. 1. At 6 months, 3 patients had remission by DAS28 and 3 had low activity. Fig. 2 shows the percentage of patients with high, moderate, low activity or remission by DAS28 at baseline and at 6, 12 and 24 months of follow-up.
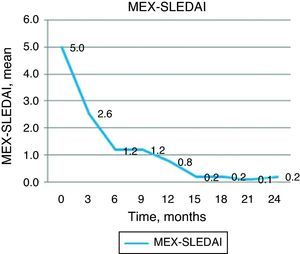
Regarding the MEX-SLEDAI score, this was reduced from 5 points at baseline to 2.56 at 3 months and 1.22 at 6 months (P<.001) and maintained this improvement at 12 (0.78) and 24 (0.22) months (Fig. 3). As already mentioned, the decision to apply a second treatment of rituximab depended on clinical activity. Eight patients showed an increase in activity by DAS28 between 9 and 12 months, so they received a second course of rituximab, and only in one patient was this necessary at 15 months. In multivariate analysis, we found a negative correlation between clinical improvement assessed by DAS28 at 12 months and the levels of anti-CCP (r=−0.794, P=.011).
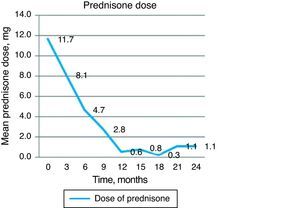
An indirect measure of clinical efficacy of the treatment is the ability to reduce steroid dose and/or discontinue it during the study. The baseline dose of prednisone in our group of patients was 11.66mg prednisone/day and was progressively decreased to 4.72mg at 6 months and 0.55 and 1.11mg/day 12 and 24 months respectively. The majority of patients did not require6 oral steroids from 12 months of treatment onward (Fig. 4).
Safety aspects: in general, treatment with rituximab was well tolerated during the study. Adverse events were divided as immediate reactions to the infusion, infectious events and serious adverse events. There were three adverse reactions in 2 patients during infusion of rituximab, which consisted of erythema and pruritus, and on all occasions forced us to temporarily stop the infusion and administer steroids and intravenous antihistamines. There were 9 non-serious infectious events in 6 patients, mainly infections of the lower urinary tract (6 cases) and infections of the upper respiratory tract (3 events), in both cases without complications. Only one patient had pneumonia, an event that was considered serious enough to require hospital management, with adequate clinical recovery. There were no deaths, malignancies or opportunistic infections during the follow up period.
DiscussionIn our study patients, rituximab treatment was effective in both joint manifestations, with significant reduction in DAS28, as in other manifestations of lupus, with improvement of MEX-SLEDAI. Clinical response was evident after 6 months, however, began to be apparent within the first 3 months of evaluation and was maintained in all patients during the monitoring phase with repeated cycles of treatment at the time clinical reactivation was shown.
Usually joint manifestations in SLE patients often respond satisfactorily to DMARDs or low dose steroids; however, in rhupus a large percentage of patients do not have good response, in many cases presenting polyarticular arthropathy with progressive structural damage.3,4,6,20,21 Among the different biologic therapy options, anti-CD20 blocking antibody rituximab may be a good choice. There are multiple reports and case series that have demonstrated the efficacy of this drug in patients with various manifestations of lupus.15 In a recent meta-analysis published by Ramos-Casals, who performed a systematic review of 188 lupus patients treated with rituximab between 2002 and 2007, therapy was effective in 91% of cases.16 Of the few randomized controlled trials that exist to date in the EXPLORER study, which included 257 patients with lupus with moderate to severe activity, there was no significant difference in the percentage of patients achieving major response (1.24 vs 15.9) or partial response (17.2 vs 12.5) in the groups treated with rituximab or placebo, respectively.12,13 Similar results were observed in the LUNAR study, involving 144 patients with lupus nephritis III or IV, in whom no significant differences could be demonstrated between rituximab and placebo.14 However, it is important to note that in both studies, rituximab response seemed better in minority groups and it also should be noted that in both studies, patients were receiving the standard treatment for lupus, making it difficult to detect differences between groups. We reported in 2010 an open study in lupus patients who received cyclophosphamide or rituximab. The results favored rituximab and although all patients had severe manifestations of the disease as a criterion for inclusion, a significant percentage had joint manifestations, which improved.22
The results of the French registry of lupus patients treated with rituximab have been recently published. The authors found an adequate clinical response in about 80% of patients, specifically, improvement occurred for articular manifestations in 72%.17 Currently, other multicenter controlled clinical studies in patients with different manifestations of lupus, such as the RING study, evaluating the efficacy of rituximab to achieve remission in lupus nephritis are underway and the results will certainly be of great value to define even better the usefulness of this biological agent.23
In patients with rhupus, although manifestations of both lupus and RA coexist, the prevalence is characteristic for the development of joint manifestations, which usually dominate the clinical picture of the disease, sometimes with mucocutaneous manifestations and much less frequently renal manifestations, serious hematological or nervous system involvement.1,3–5 This was no different in our patients, in which the expression of joint disease was unwieldy, with general or mucocutaneous manifestations associated with low scores explaining MEX-SLEDAI, which, however, improved during the course of the study.
We also described the relationship between the severity of joint manifestations in patients with rhupus and positivity for anti-CCP;5,24–26 hence the negative correlation we found between the titles of these antibodies and clinical response is entirely explicable.
While recognizing the limitations of our work, which included a small number of patients with rhupus due to the very low prevalence of this condition1 since it is an open uncontrolled study, we believe that the results obtained are encouraging and, to some extent, expected, and considering past experience, which is extensive, treatment with rituximab in patients with RA and reports of improvement in many manifestations of SLE, place rituximab as a very suitable drug for the management of patients with lupus and even rhupus and prominent joint manifestations.
Ethical ResponsibilitiesProtection of People and AnimalsThe authors declare that procedures conformed to the ethical standards of the committee responsible for human experimentation and were in accordance with the World Medical Association Declaration of Helsinki.
Data ConfidentialityThe authors declare that they have followed the protocols of their workplace on the publication of data from patients and all patients included in the study have received sufficient information and gave their written informed consent to participate in this study.
Right to Privacy and Informed ConsentThe authors have obtained informed consent from patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflict of InterestAndrade-Ortega: speaker for Roche, Bristol, Pfizer, Merck.
Irazoque-Palazuelos: Advisory board and/or speaker for Roche, Bristol, Pfizer, Janssen, Merck.
Muñoz-López: speaker for Sanofi, Pfizer.
Don Pablo Rosales: lecturer for Pfizer.
Please cite this article as: Andrade-Ortega L, et al. Eficacia y tolerabilidad de rituximab en el tratamiento de pacientes con rhupus. Reumatol Clin. 2013;9:201–5.