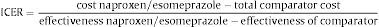To assess, from the perspective of the National Healthcare System, the efficiency of a fixed-dose combination of naproxen and esomeprazole (naproxen/esomeprazole) in the treatment of osteoarthritis (OA) compared to other NSAID, alone or in combination with a proton pump inhibitor (PPI).
MethodsA Markov model was used; it included different health states defined by gastrointestinal (GI) events: dyspepsia, symptomatic or complicated ulcer; or cardiovascular (CV) events: myocardial infarction, stroke or heart failure. The model is similar to the one used by NICE in its NSAID evaluation of OA published in 2008.
The total costs (€, 2012), including drug and event-related costs, and the health outcomes expressed in quality-adjusted life years (QALY) were estimated in patients with increased GI risk, aged 65 or over, for a 1-year time horizon and a 6-month treatment with celecoxib (200mg/day), celecoxib+PPI, diclofenac (150mg/day)+PPI, etoricoxib (60mg/day), etoricoxib+PPI, ibuprofen (1800mg/day)+PPI, naproxen (1000mg/day)+PPI or naproxen/esomeprazole (naproxen 1000mg/esomeprazole 40mg/day). The selected PPI was omeprazole (20mg/day).
ResultsNaproxen/esomeprazole was a dominant strategy (more effective and less costly) compared to celecoxib, etoricoxib and diclofenac+PPI. Celecoxib+PPI and etoricoxib+PPI were more effective.
Considering a cost-effectiveness threshold of € 30000 per additional QALY, naproxen/esomeprazole was cost-effective compared to ibuprofen+PPI and naproxen+PPI with incremental cost-effectiveness ratios (ICER) of € 15154 and € 5202 per additional QALY, respectively.
ConclusionsA fixed-dose combination of naproxen and esomeprazole is a cost-effective, and even dominant, alternative compared to other options in OA patients with increased GI risk.
Evaluar, desde la perspectiva del Sistema Nacional de Salud, la eficiencia de la combinación a dosis fija de naproxeno y esomeprazol (naproxeno/esomeprazol) en artrosis frente a otros AINE en monoterapia o combinados con un inhibidor de la bomba de protones (IBP).
MétodosSe empleó un modelo de Markov con estados de salud definidos por episodios gastrointestinales (GI): dispepsia, úlcera péptica sintomática o complicada; o cardiovasculares (CV): infarto agudo de miocardio, ictus o insuficiencia cardiaca. El modelo es semejante al utilizado por el NICE en su evaluación de AINE en artrosis publicada en 2008.
Se estimaron, en un horizonte temporal de 1 año (ciclos de 3 meses), los costes totales (€, 2012), incluyendo coste farmacológico y de manejo de episodios, y los resultados en salud, expresados en años de vida ajustados por calidad (AVAC), en pacientes mayores de 65 años con riesgo GI aumentado, tras 6 meses de tratamiento con celecoxib (200mg/día), celecoxib+IBP, diclofenaco (150mg/día)+IBP, etoricoxib (60mg/día), etoricoxib+IBP, ibuprofeno (1.800mg/día)+IBP, naproxeno (1.000mg/día)+IBP o naproxeno/esomeprazol (naproxeno 1.000mg/esomeprazol 40mg/día). El IBP fue omeprazol (20mg/día).
ResultadosNaproxeno/esomeprazol resultó dominante (más efectivo y menor coste) respecto a celecoxib, etoricoxib y diclofenaco+IBP. Celecoxib+IBP y etoricoxib+IBP fueron más efectivos.
Considerando un umbral de 30.000€/AVAC adicional, naproxeno/esomeprazol resultó coste-efectivo respecto a ibuprofeno+IBP y naproxeno+IBP con valores de relación coste-efectividad incremental de 15.154€ y 5.202€/AVAC adicional, respectivamente.
ConclusionesLa combinación a dosis fijas de naproxeno y esomeprazol en pacientes con artrosis y riesgo GI aumentado es una alternativa coste-efectiva e incluso dominante frente a otras opciones.
Osteoarthritis is the most common joint disease and a major cause of functional disability and impaired quality of life.1 It is one of the most common reasons for visits to primary care and has a high socioeconomic impact2 with an estimated annual cost of € 1502 per patient, resulting in a total expense of 511billion euros a year in Spain.3 It occurs in all populations and its incidence increases with age. It is estimated to affect 85% of the elderly population and disables 10% of people over 60 years, mainly women.2 The prevalence of knee and hands osteoarthritis in the Spanish population was estimated at 10.2% and 6.2%, respectively.1
The goals of osteoarthritis treatment are to relieve pain, improve joint function and delay disease progression in terms of structural joint damage, preventing the toxic effects of treatment. In choosing the therapeutic strategy, clinicians can turn to the recommendations of the European League Against Rheumatism (EULAR),4 and the American College of Rheumatology (ACR),5 as well as the consensus documents of the Spanish Society of Rheumatology (SER)6 or to the guidelines of the Osteoarthritis Research Society International (OARSI).7
Most of the therapeutic goals can be achieved by treatment with various non-selective nonsteroidal anti-inflammatory drugs (NSAIDs). However, NSAID use is frequently associated with gastrointestinal disorders (GI) which can range from mild discomfort to severe adverse events such as perforations and bleeding; this is associated with a high consumption of health resources.8 Concomitant administration of proton pump inhibitors (PPIs) has shown an inverse relationship with the development of GI episodes, strongly influenced by the adherence to PPIs.9
The introduction of selective cyclooxygenase inhibitors 2 (ICOX-2) of similar efficacy provided an interesting alternative for improving the toxicity profile in terms of GI events compared to traditional NSAIDs. However, their widespread use has been associated to an increase of cardiovascular events (CV), some of which also involve traditional NSAIDs, with the possible exception of naproxen, which has not been associated with increased cardiovascular events.10
Therefore, strategies can be employed with NSAID use, both traditional and ICOX-2 of similar efficacy but different safety profiles, which affect the quality of life related to the health of patients with osteoarthritis.
The fact that health care resources are limited requires that prescription be an act that considers the most effective among the available drugs and selects the most effective in treating the disease in question, prescribing that which enables a lower incremental cost per additional unit of effectiveness.
The fixed dose combination of naproxen and esomeprazolea (naproxen/esomeprazole) combines the efficiency of naproxen as an NSAID, with a lower incidence of NSAID-associated ulcers and better tolerated in the upper digestive tract, due to its association to esomeprazole, a PPI.11 Its efficacy in osteoarthritis is equivalent to ICOX-2 and has proven to maintain its profile of GI and CV safety, even in the long term.12
The objective of this analysis was to conduct an assessment of the efficiency of naproxen/esomeprazole as an alternative therapy in patients with osteoarthritis compared to other NSAIDs available in Spain, both traditional and ICOX-2, alone or administered with a PPI.
Materials and MethodsModel StructureWe used a Markov model, developed in Microsoft Excel 2007, to simulate the course of the disease in a hypothetical cohort of patients passing through different states of health. These models are commonly used in simulations of chronic diseases. The health states should be mutually exclusive, so the patient at all times can only be in one of these states, remaining for a uniform period of time, called a cycle. At the end of each cycle the patient may pass or move to another state according to transition probabilities.
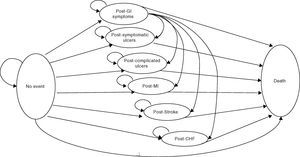
In this case eight health states were created among which patients could move in defined cycles of 3 months. From the initial “without incident” state, the patient evolves to the “death” state or 6 other states derived from the appearance of a clinical event: GI-dyspepsia, symptomatic or complicated ulcer or ulcer-CV-myocardial infarction (MI), stroke, or congestive heart failure (CHF) (Fig. 1).
Except for dyspepsia, the remaining episodes were considered serious. After a severe episode, the patient remained in the corresponding post-episode state for the rest of the simulation or until transition to the absorbing health state (death). However, to consider the fact that, in clinical practice, a patient may experience later episodes, and that the probability of occurrence of these events is highest in patients who have had a previous episode, the cost and the associated usefulness of each severe postepisode state were weighted to take into account other possible future episodes. Patients with dyspepsia could remain in this state for the rest of the simulation, resulting in a serious episode with the implications described, or die.
The profile of the population sampled in this model reflects a patient over 65 years of age with osteoarthritis and increased GI risk, defined as a history of ulcer (complicated or uncomplicated) in the upper GI tract.
In a time horizon of one year, total costs (including the cost of drug treatment and the cost of managing clinical events) and health outcomes at 6 months of treatment with any of the following strategies were estimated: celecoxib, PPI+celecoxib, diclofenac+PPI, etoricoxib, etoricoxib+PPI+PPI ibuprofen, naproxen+PPI or naproxen/esomeprazole. The PPI of choice was omeprazole, being the most used and the least costly, at doses of 20mg/day. The duration and dosage considered for each alternative represented the most frequently used treatment by me in clinical practice for the treatment of patients with the profile described (Table 1).
Dosage and Cost of Therapeutic Alternatives.
| Therapeutic alternatives | Mode of administration | PVP-tax cost (container) |
| Celecoxib | 200mg daily | € 34.35a (30 tablets, 200mg) |
| Diclofenac | 150mg daily | € 1.65b (40 tablets, 50mg) |
| Etoricoxib | 60mg daily | € 30.06c (28 tablets, 60mg) |
| Ibuprofen | 1800mg daily | € 1.97b (40 tablets, 600mg) |
| Naproxen | 1000mg daily | € 4.34b (40 tablets, 500mg) |
| Naproxen/esomeprazole | 1000/40mg daily | € 23.71d (60 tablets, 500/20mg) |
| Paracetamol | 3000mg daily | € 2.79b (40 tablets, 1000mg) |
| Omeprazole | 20mg daily | € 2.42b (28 tablets, 20mg) |
After 6 months of treatment, or if severe episode occurred, involving permanent discontinuation of the NSAIDs, the model assumed that patients went on to receive treatment with paracetamol (3000mg/day) for the rest of the simulation.13 In the absence of evidence to the contrary, it was assumed that the effect of the treatment received did not persist after its end.14 The development of GI symptoms (dyspepsia) involved incorporating PPI to the treatment received in cases of therapeutic strategies which did not include PPI (celecoxib and etoricoxib).
Adherence to established strategies were evaluated in terms of adherence to PPI. This analysis found that within 6 months of treatment, adherence was 69%.9
Type of AnalysisThe efficiency of naproxen/esomeprazole was established by its incremental cost effectiveness (ICER) for each of the other relation strategies evaluated according to the following formula:
The unit of effectiveness used were adjusted life years (QALYs). QALYs combine into a single value, quantity, and quality of life, and are calculated by multiplying survival by the usefulness value. The usefulness is a parameter representing the preference of patients for a given health condition, taking into account the effect on their quality of life. The value of 1 means a state of perfect health, and the value of 0 equals death.
Model ParametersThe probability of moving to any of the states considered are derived from the risk of GI and CV events and associated mortality. The odds of each episode appearing were obtained from the evaluation conducted by the National Institute for Health and Care Excellence (NICE),13 using the same premise to adjust the dosages to: a reduction of 50% of the dose involved a 25% reduction in risk of developing an episode.13,14 For this analysis it was assumed that the risk of GI episodes with naproxen/esomeprazole is equivalent to naproxen+PPI separately if there is 100% adherence to the PPI, and that the risk of CV events is equivalent to naproxen. Table 2 lists the probabilities used for development of the first episode. PPI administration along with the therapeutic alternatives involved reduced the probability of GI episodes compared to monotherapy.13,14 This model considers that patients with a history of GI episodes have an increased risk of a new GI episode.15 Similarly, the existence of previous episodes of MI, stroke or heart failure was associated with an increased likelihood of having a CV event. Relationship between the level of adherence and the risk of GI events was determined using the same methodology as that used by other authors9 calculating the increased risk of GI episodes per 10% loss of adhesion (Table 3). The model considered both overall mortality by age of the Spanish population, obtained from the tables of the National Statistics Institute (INE) and the mortality associated with GI complicated (complicated ulcers), MI, stroke or congestive heart failure episodes, whose values were obtained from the literature.16,17
Probability of Development of the First Clinical Episode.
| Therapeutic alternatives | Clinical episodes | |||||
| Dyspepsia | Ulcer | Complicated ulcer | MI | Stroke | Congestive heart failure | |
| Celecoxib | 0.12450 | 0.00090 | 0.00050 | 0.00150 | 0.00020 | 0.00040 |
| Celecoxib +PPI | 0.03113 | 0.00023 | 0.00013 | 0.00150 | 0.00020 | 0.00040 |
| Diclofenac+PPI | 0.10991 | 0.00062 | 0.00039 | 0.00108 | 0.00072 | 0.00024 |
| Etoricoxib | 0.16307 | 0.00120 | 0.00093 | 0.00133 | 0.00093 | 0.00053 |
| Etoricoxib+PPI | 0.04077 | 0.00030 | 0.00023 | 0.00133 | 0.00093 | 0.00053 |
| Ibuprofen+PPI | 0.06564 | 0.00089 | 0.00044 | 0.00180 | 0.00072 | 0.00108 |
| Naproxen+PPI | 0.07352 | 0.00118 | 0.00037 | 0.00069 | 0.00091 | 0.00103 |
| Naproxen/esomeprazole | 0.07352 | 0.00118 | 0.00037 | 0.00069 | 0.00091 | 0.00103 |
| Paracetamola | 0.12720 | 0.00040 | 0.00020 | 0.00060 | 0.00030 | 0.00010 |
MI, acute myocardial infarction; PPI, proton pump inhibitor; CHF, congestive heart failure.
Relationship Between PPI Adherence and Risk of GI Episodes.
| Therapeutic strategies | Increase (%) in the risk of GI episode for every 10% loss of adherence to PPI level | ||
| Dyspepsia | Symptomatic ulcer | Complicated ulcer | |
| With COX-2 inhibitors (celecoxib+PPI or etoricoxib+PPI) | 8.8 | 10.5 | 8.1 |
| Nonselective NSAID (diclofenac+PPI or ibuprofen+PPI or naproxen+PPI) | 14.9 | 14.9 | 14.9 |
NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitor; GI, gastrointestinal.
Different values were considered useful, depending on the age, the underlying pathology and clinical events.14 In the absence of specific data for Spanish population, earnings by age were obtained from a health survey performed in the UK.14 As an item associated with osteoarthritis in the absence of clinical episodes, a meta-analysis results of the WOMAC scale showed no differences between NSAIDs and ICOX-2.13,14 Earnings per development of clinical events were obtained from the literature14 (Table 4).
Utilities and Costs per Episode and Health State.
| Health status | Utilities mean (95% CI) | Cost of episode management/cost of state |
| No episode | 1.00 | NA |
| GI symptoms (dyspepsia) | 0.73 (0.63–0.84) | € 324.9620 |
| Symptomatic ulcer | 0.55 (0.47–0.65) | € 2884.98 (average DRG177 and 178)19 |
| Postulcer symptomatic | 0.98 | Equivalent to dyspepsia |
| Complicated ulcer | 0.46 (0.37–0.56) | € 3430.30 (average DRG174, 175 and 176)21 |
| Postulcer complicated | 0.98 | Equivalent to dyspepsia |
| MI | 0.37 (0.28–0.47) | € 5595.63 (average DRG121 and 122)21 |
| Post-MI | 0.88 (0.78–0.98) | € 259.5321 |
| Stroke | 0.35 (0.25–0.45) | € 4964.48 (DRG14 and 810)21 |
| Poststroke | 0.71 (0.61–0.80) | € 107.4422 |
| CHF | 0.71 (0.61–0.80) | 3575.43 (DRG127)21 |
| Post-CHF | 1.00 | Equivalent post-MI |
| Death | 0.00 | € 0.00 |
GI, gastrointestinal; DRG, diagnostic related group; MI, acute myocardial infarction; CI, confidence interval; CHF, congestive heart failure; NA, not applicable.
The analysis was conducted from the perspective of the Spanish National Health System, including only the costs associated with pharmacological treatment and management derived from clinical episodes. The cost of drug therapies for each 3 months, 91 established in days, was calculated taking into account the doses of study, from the retail price (RRP-IVA) and applying the appropriate 7.5% deduction established in the Royal Decree-Law 8/2010. For generic drugs and in which this deduction does not apply, we chose the lowest-priced generic. The costs of drugs were obtained from the Books of General Council of Official Colleges of Pharmacists.18 The costs of managing clinical episodes, and those associated with the state of health after each episode, were obtained from Diagnosis Related Groups (DRGs) national aggregates19 or the literature.20–22 In the absence of published data on the costs associated with symptomatic postulcer and complicated postulcer states considered that these were equivalent to the cost of management of dyspepsia.20 For the post-congestive heart failure state, the cost was assumed to be22 equivalent to the post-MI state, consistent with the premise adopted by NICE in previous assessments.23Table 4 includes the costs used in the analysis. All costs in euros are presented as 2012 euros, after correction of cost data with the consumer price index (CPI) provided by the INE in appropriate cases.
As the horizon of the analysis was one year, no discount rate was applied to costs or health effects.
Sensitivity AnalysisDeterministic sensitivity analyzes were performed to identify the influence on the results of variations in the following parameters:
- -
Duration of treatment: 3 months, since there is variability in the length, being sometimes shorter (intermittent symptoms).
- -
Dosage of ibuprofen: due to increased variety in relation to the dose used in clinical practice for this population, and since some guidelines recommend dosages reaching even 2400mg/day, an analysis was performed based on the maximum dose.24,25
- -
Price of celecoxib: due to the possible availability of celecoxib generic version throughout 2013, its price was reduced by 40%.
- -
Costs of managing events (±10%); because these parameters were susceptible to change.
- -
Earnings per episode: modified with the upper and lower ends of the 95% CI (Table 4).
Additionally, a probabilistic sensitivity analysis (PSA) was performed to change randomly and multivariate the values of the parameters, thus retaining the possibility of extreme variations from the baseline case and allowing to estimate, due to the high number of simulations, the robustness of the results based on those considered outliers. 10000 Monte Carlo simulations were performed where the values of the parameters are changed simultaneously according to the following functions: beta probability distribution, adherence, mortality and utilities, log normal distribution and gamma distribution risks relating to episode cost management and cost of health states.
ResultsAt the end of the 12-month simulation and 6 months of treatment, naproxen/esomeprazole resulted in a (more effective and less associated cost) than the celecoxib, etoricoxib and diclofenac+PPI dominant strategy. Whereas an efficiency threshold € 30000/QALY is an acceptable additional value of willingness to pay, naproxen/esomeprazole would be cost-effective regarding ibuprofen+PPI and naproxen+PPI strategy. Celecoxib+PPI and etoricoxib+PPI were more effective than naproxen/esomeprazole strategies, which prevented the calculation of the ICER of naproxen/esomeprazole with respect to them with the proposed formula for this calculation. These results are detailed in Table 5.
Results of the Baseline Case. Years of Quality-adjusted Life Years (QALY), Costs and Incremental Cost-effectiveness Ratio (ICER) of Naproxen/Esomeprazole vs Other Alternatives.
| Therapeutic strategies | QALY | Total cost (€) | ICER (€/QALY additional naproxen/esomeprazole vs) |
| Naproxen/esomeprazole | 0.5911 | € 662.71 | |
| Celecoxib | 0.5765 | € 843.35 | Naproxen/esomeprazole is dominant |
| Etoricoxib | 0.5635 | € 960.06 | Naproxen/esomeprazole is dominant |
| Diclofenac+PPI | 0.5708 | € 674.67 | Naproxen/esomeprazole is dominant |
| Ibuprofen+PPI | 0.5870 | € 599.49 | € 15154.20/QALYa |
| Naproxen+PPI | 0.5843 | € 627.19 | € 5201.65/QALYa |
| Celecoxib+PPI | 0.5996 | € 659.42 | NAb |
| Etoricoxib+PPI | 0.5946 | € 699.55 | NAb |
NA, not applicable.
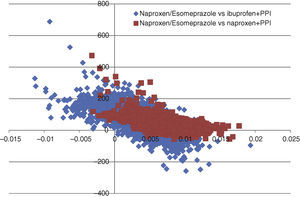
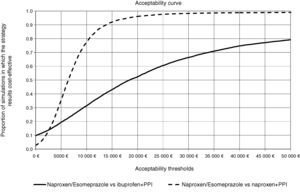
In all deterministic sensitivity analysis performed (Table 6), the results of naproxen/esomeprazole remained: dominant vs celecoxib and etoricoxib, cost-effective compared to ibuprofen+PPI and naproxen+PPI and showing no change vs diclofenac+PPI, becoming cost-effective in reducing treatment to 3 months and reducing the costs of episodes by 10%. We performed two separate PSA vs the options for naproxen/esomeprazole and that proved cost-effective in the baseline case, ibuprofen+PPI and naproxen+PPI. In the PSA, after 10000 simulations, the average ICER, naproxen/esomeprazole stood at 18436 and € 6367/additional QALY vs+ibuprofen+PPI and naproxen+PPI respectively, 66.4% and 98.3% of cases were considered cost-effective (below the threshold of € 30000/additional QALY), respectively for each strategy (Figs. 2 and 3).
Results of Deterministic Sensitivity Analysis.
| Therapeutic strategies | Incremental QALY (naproxen/esomeprazole vs) | Total incremental cost (€) (naproxen/esomeprazole vs) | ICER (additional €/QALY naproxen/esomeprazole vs) |
| Duration of treatment: 3 months | |||
| Celecoxibaa | 0.0075 | −99.97 | Naproxen/esomeprazole is dominant |
| Etoricoxibaa | 0.0139 | −156.58 | Naproxen/esomeprazole is dominant |
| Diclofenac+PPI | 0.0096 | 12.83 | € 1342.63/QALY |
| Ibuprofen+PPI | 0.0017 | 48.31 | € 28353.52/QALY |
| Naproxen+PPI | 0.0030 | 33.17 | € 11169.24/QALY |
| Cost of episode handling: 10% increase | |||
| Celecoxibaa | 0.0147 | −190.74 | Naproxen/esomeprazole is dominant |
| Etoricoxiba | 0.0276 | −321.00 | Naproxen/esomeprazole is dominant |
| Diclofenac+PPI | 0.0203 | −25.92 | Naproxen/esomeprazole is dominant |
| Ibuprofen+PPI | 0.0042 | 57.44 | € 13768.92/QALY |
| Naproxen+PPI | 0.0068 | 28.51 | € 4175.96/QALY |
| Episodes handling costs: 10% off | |||
| Celecoxiba | 0.0147 | −170.54 | Naproxen/esomeprazole is dominant |
| Etoricoxiba | 0.0276 | −273.70 | Naproxen/esomeprazole is dominant |
| Diclofenac+PPI | 0.0203 | 2.00 | € 98.55/QALY |
| Ibuprofen+PPI | 0.0042 | 68.99 | € 16539.49/QALY |
| Naproxen+PPI | 0.0068 | 42.52 | € 6227.33/QALY |
| Dose of ibuprofen: 2400mg/day | |||
| Ibuprofen+PPI | 0.0073 | 14.64 | € 1995.29/QALY |
| Price celecoxib: estimate of the generic version price (40% reduction on current price) | |||
| Celecoxibaa | 0.0147 | −90.23 | Naproxen/esomeprazole is dominant |
| Earnings per episode: upper limit of 95% CI | |||
| Celecoxibaa | 0.0086 | −180.64 | Naproxen/esomeprazole is dominant |
| Etoricoxiba | 0.0166 | −36.85 | Naproxen/esomeprazole is dominant |
| Diclofenac+PPI | 0.0120 | −11.96 | Naproxen/esomeprazole is dominant |
| Ibuprofen+PPI | 0.0026 | 63.21 | € 24153.89/QALY |
| Naproxen+PPI | 0.0042 | 35.52 | € 8515.03/QALY |
| Earnings per episode: lower limit of 95% CI | |||
| Celecoxibaa | 0.0202 | −180.64 | Naproxen/esomeprazole is dominant |
| Etoricoxiba | 0.0376 | −36.85 | Naproxen/esomeprazole is dominant |
| Diclofenac+PPI | 0.0279 | −11.96 | Naproxen/esomeprazole is dominant |
| Ibuprofen+PPI | 0.0056 | 63.21 | € 11322.89/QALY |
| Naproxen+PPI | 0.0092 | 35.52 | € 3846.38/QALY |
QALY, quality-adjusted life years; ICER, incremental cost-effectiveness ratio.
Acceptability curve of naproxen/esomeprazole. The acceptability curve reflects certain thresholds for willingness to pay (from 0 to 50000 € represented on the horizontal axis), the proportion (on the vertical axis) of the 10000 ICER values obtained in the PSA that would be less than that determined threshold, considered cost effective.
NSAIDs, both non-selective as well as those selective for inhibition of COX-2, are effective therapeutic alternative in the treatment of osteoarthritis. However, tolerability and GI and CV safety factors associated with its use in certain populations contraindicate their use or require appraisal of the individual profile of benefit/risk for each patient. Thus, although ICOX-2 reduces the risk of gastrointestinal complications, they have not shown, on occasion, a suitable CV safety profile. Conversely, naproxen is a therapeutic agent with a good CV safety profile at doses of 500mg/12h, but does not have this profile regarding the digestive tract. It seems logical that the latter risk reduction (with the addition of esomeprazole) would give naproxen a role in the symptomatic treatment of osteoarthritis, and that this would be an efficient therapeutic choice.
The many potential variables in the GI and CV risk for each patient makes assignment of the best therapeutic strategy based on existing resources difficult, so that an indicator such as ICER, which combines clinical and economic outcomes relative to different therapies, is very interesting.
The ICER of naproxen/esomeprazole was favorable, even in the analysis of sensitivity compared to other options, except for the treatment ICOX-2+PPI. The initial advantage of ICOX-2 came from the lack of need, due to their lower rate of GI events, of concomitant use of a PPI, although in recent times there has been a change in clinical practice, with the recommendation of using ICOX-2+PPI in elderly patients with a history of gastrointestinal bleeding and the presence of multiple risk factors.26
The results are important from the point of view of clinical practice. The patient with osteoarthritis is usually elderly and has chronic multiple comorbidities including pathologies, usually CV and include gastrointestinal manifestations. Numerous studies have stratified these risks in said population and have established indication for NSAIDs in order to minimize the impact of adverse events.26,27 The availability of a fixed combination of a highly effective NSAID with excellent CV profile, such as naproxen, along with a powerful PPI, might be able to mitigate its digestive impact by itself, according to the authors, becoming a good alternative. The analysis performed in this study further corroborates, from the pharmacoeconomic point of view, its favorable clinical medicine perspective.
This work is not without some limitations, some inherent to the theoretical association or to the use of decision analytic models, simulations that may not be an accurate reflection of clinical practice.
The validity and quality of decision analytical studies lie in their programming, as well as in the assumptions made and the values for the parameters included under consideration. In the development of economic evaluations it is crucial that the model has clinical sense and that the data comes from reliable and verifiable, preferably published sources.
This model was developed with the baseline assumption of equivalence in terms of efficacy of all treatments included, so that the differences between the evaluated strategies lie in the differences in costs and rates of adverse events. The decision on inclusion of clinical episodes was made considering the clinically relevant and data availability required13 for the episodes. The equivalence in efficacy, if not exact, could be a bias of the design, but in the absence of proven evidence from controlled head-to-head trials it has to be understood as a reasonable premise.13 The adjustment made in relation to the dose and the occurrence of clinical events that are handled in the model represents a limitation, although the methodology is consistent with that used in other studies which concluded that the uncertainty associated with this parameter did not influence the14 results.
In the absence of specific data concerning Spanish population it was necessary to use utility values per age obtained from the UK population. Utilities, associated with cultural factors, may differ even between countries in the same environment, but the sensitivity analysis, changing the values of earnings per episode, showed no influence of this parameter on the results.
Additionally, the fact that in each 3 month cycle the patient may only experience one GI or CV episode must be considered. This assumption may not be entirely realistic, but its adoption was necessary to build the model, also considering the generation of no significant impact on the results.13
The limitations described were offset by conservative assumptions and tested in sensitivity analyzes, showing no significant influence on the meaning of the results.
There are several publications on economic evaluations of treatments used in osteoarthritis, both internationally and in the14 Spanish context,20,28,29 although, to the authors’ knowledge, this is the first cost-utility analysis of naproxen/esomeprazole over other ICOX-2 NSAIDs or adapted to Spain, also individualized for each NSAID, instead of treating all NSAIDs as a group.
The results obtained from the analysis of a hypothetical cohort of patients with a decision analytic model suggest that naproxen/esomeprazole is an appropriate therapeutic option that is dominant over other strategies available, such as celecoxib, etoricoxib and diclofenac+PPI; and also using the standard reference threshold € 30000/additional QALY,30,31 showing it as a cost-effective strategy against both the option prescribed in clinical practice (ibuprofen+PPI) compared to the option of separate single-components (naproxen+PPI).
Ethical ResponsibilitiesProtection of human subjects and animals in researchThe authors declare that experiments have not been done on humans or animals.
Confidentiality of dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of InterestIO and MAC are PORIB employees, a consultant agency specialized in the area of economic evaluation of health technologies, have received unconditional funding for this analysis from AstraZeneca.
JSC and MC are employees of AstraZeneca.
JT, AL and JLZ have worked as clinical experts in the validation of the values of the parameters used in the analysis, and therefore claim to have received unconditional funding from AstraZeneca, which never influenced the participation and results obtained.
The authors thank the reviewers of Reumatología Clínica for the comments provided during the review of the manuscript.
Please cite this article as: Capel M, Tornero J, Zamorano JL, Oyagüez I, Casado MÁ, Sánchez-Covisa J, et al. Eficiencia de la combinación naproxeno/esomeprazol para el tratamiento de la artrosis en España. Reumatol Clin. 2014;10:210–217.
This fixed dose combination is marketed in Spain as modified-release tablets containing naproxen with enteric coating and film-coated esomeprazole (as magnesium trihydrate).