The exposure to artificial gravity (AG) through human centrifugation is the basis of the treatment called gravity therapy (GT), in which the mechanical stimulation over the vessel wall, induces the synthesis and release of prostacyclin. It has been used for more than four decades in Uruguay in the treatment of different vascular-based pathologies. In patients with systemic sclerosis (SSc) it has shown good benefits and excellent safety profile over the years. However, there is a lack of knowledge in the scientific community about GT and its results.
ObjectiveTo evaluate the effectiveness of GT in cutaneous and vascular involvement, in the quality of life and functional capacity and its safety profile in patients with SSc.
MethodologyIt is a descriptive and retrospective study of patients with SSc assisted in an autoimmunity center in Montevideo, treated with GT in the last 10 years.
ResultsFifty patients were included, 48 women (96%) and 2 men (4%) with a mean age of 62 ± 12 years. The mean time of evolution of SSc at the time of inclusion in the study at the beginning of GT was 6.8 ± 3.2 years and 2.8 ± 3.2 years respectively. After GT, a significant improvement in the modified Rodnan skin score (mRSS) was observed (pre-GT 19.2 ± 8.7 vs. post-GT 5.4 ± 5.0, p < 0.05), which was not related to the time of disease progression at the beginning of GT nor to the skin extension or immunological profile. The degree of improvement post-GT was related to a higher initial mRSS (R = 0.84, p < 0.05). Also, a significant improvement was observed in the number of patients with puffy fingers (pre-GT 50% vs. post-GT 20% patients, p < 0.05), but not in telangiectasias, pitting scars or sclerodactyly. The severity of Raynaud's phenomenon significantly decreased (pre-GT: grade 3–4, 43/48 (89.6%) patients vs. post-GT: grade ≤2, 42/47 (89.4%) patients, p < 0.05) as well as the vascular pain measured with VAS (0–10 scale) (pre-GT: 7.6 ± 2.2 vs. post-TG: 1.4 ± 1.2, p < 0.05). The healing of digital ulcers was also recorded. Regarding the results reported by patients, 97% reported improvement in the quality of life and 89.5% improvement in the ability to carry out activities of daily living. No significant adverse effects were recorded.
ConclusionsGT improved cutaneous and vascular involvement, the quality of life and the functional capacity in patients with SSc with an excellent safety profile. Randomized, controlled clinical trials are needed to corroborate these observational results.
La exposición a la gravedad artificial (GA), a través de la centrifugación humana, constituye la base del tratamiento denominado terapia gravitacional (TG), mediante el cual se produce un estímulo mecánico sobre la pared vascular induciendo la síntesis y liberación de prostaciclina. Se usa desde hace más de cuatro décadas en Uruguay en el tratamiento de enfermedades de base vascular. En la esclerosis sistémica (ES) ha demostrado gran beneficio y excelente perfil de seguridad. Sin embargo, existe un desconocimiento en la comunidad científica acerca de la TG y de sus resultados.
ObjetivoEvaluar la eficacia de la TG en la afección cutánea y vascular, calidad de vida y capacidad funcional y su perfil de seguridad en pacientes con ES.
MetodologíaEstudio descriptivo y retrospectivo de pacientes con ES asistidos en un centro de autoinmunidad de Montevideo, tratados con TG en los últimos 10 años.
ResultadosSe incluyeron 50 pacientes, 48 mujeres (96%) y 2 hombres (4%) con una edad media de 62 ± 12 años. El tiempo medio de evolución de la ES al momento de inclusión en el estudio y al iniciar la TG, fue de 6.8 ± 3.2 años y de 2.8 ± 3.2 años respectivamente. Post-TG se observó una mejoría significativa del puntaje modificado de Rodnan (mRSS) (pre-TG 19.2 ± 8.7 vs. post-TG 5.4 ± 5.0, p < 0.05), que no se relacionó con el tiempo de evolución de la enfermedad al inicio de la TG, extensión cutánea o perfil inmunológico. El grado de mejoría post-TG se correlacionó con un mayor mRSS inicial (R = 0.84, p < 0.05). Se observó mejoría significativa del número de pacientes con puffy fingers (pre-TG 50% vs. post-TG 20% pacientes, p < 0.05), pero no en telangiectasias, pitting scars o esclerodactilia. La gravedad del fenómeno de Raynaud disminuyó significativamente, pre-TG: grado 3–4, 43/48 (89.6%) pacientes vs. post-TG: grado ≤2, 42/47 (89.4%) pacientes, p < 0.05, así como también el dolor vascular medido por EVA (escala 0–10) (pre-TG: 7.6 ± 2.2 vs. post-TG:1.4 ± 1.2, p < 0.05). También se registraron casos de curación de úlceras digitales. En cuanto a los resultados reportados por los pacientes, 97% refirieron mejoría en calidad de vida y 89.5% mejoría en capacidad para realizar actividades de la vida diaria. No se registraron efectos adversos significativos.
ConclusionesLa TG mejoró el compromiso cutáneo y vascular, la calidad de vida y la capacidad funcional en pacientes con ES con excelente perfil de seguridad. Se necesitan ensayos clínicos controlados y aleatorizados para corroborar estos resultados observacionales.
Systemic sclerosis (SSc) is a chronic autoimmune disease of unknown aetiology. Pathogenetically, it is characterised by immune dysregulation, vasculopathy and excess collagen deposition, which determines cutaneous fibrosis and multi-organ involvement.1 Microvascular structural damage is responsible for vascular manifestations such as Raynaud's phenomenon (RP), telangiectasias, myocardial involvement, antral vascular ectasia, pulmonary arterial hypertension, digital ulcers (DU) and scleroderma renal crisis.2 Vasculopathy is secondary to the immunoinflammatory process, which determines endothelial dysfunction and damage.2,3 This is expressed by the decrease in the synthesis and release of prostacyclin and nitric oxide (NO), causing vasospasm, in situ thrombosis and intimal proliferation, which contributes to fibrosis and vascular wall stiffness with stenosis. In addition to the loss and obliteration of capillaries, there is an absence of new vessel formation. In SSc, angiogenesis is severely compromised.2–5 Abnormalities in microcirculation can be detected early by periungual capillaroscopy.
SSc impairs patients’ quality of life and determines socio-economic personal and health repercussions. There is currently no curative treatment. The latter is usually symptomatic, and in some cases it achieves patient stabilisation for a variable period of time. Some drugs can have important side effects, which does not favour adherence to treatment and can impact on the patients’ quality of life. Furthermore, some pharmacological treatments are expensive or are unavailable in Uruguay (e.g., intravenous prostacyclin analogues).
Exposure to artificial gravity (AG) through human centrifugation is the basis of the treatment called gravity therapy (GT) and has been used in Uruguay for more than 40 years. It has been applied for the treatment of vascular pathologies and RP, but also in lymphoedema, complex regional pain syndrome, and for physical rehabilitation of other medical conditions, with successful results.6–18
Isasi M.E. and Isasi E.S. showed that the frictional vector of the haemodynamic forces generated by GT, through human centrifugation, constitutes a vascular mechanical stimulus that induces the synthesis and release of vasodilatory substances such as prostacyclin and possibly NO.6,9,15,18 In addition to sustained vasodilation, it increases vascular endothelial turnover and collateral circulation.7–10 Since the vascular endothelium is sensitive to mechanical stress/stimulation during hypergravity, it has been speculated that endothelial cells would play a central role in the observed therapeutic effects.6,13,19,20 Also the randomised controlled study by Mitropoulos et al.21 suggests that mechanical/biophysical stimulation has the potential to improve microvascular endothelial dysfunction in SSc patients. In this regard, it is possible that the haemodynamic forces generated by GT induce the synthesis and release of other biologically active substances, growth factors and cytokines and modulate endothelial gene expression, in analogy to the effects of shear stress on the endothelium.6
GT has been used in Uruguay since 1990 for the treatment of SSc and has been shown to improve RP, microcirculation, DUs and cutaneous sclerosis.11,12,22,23 In the studies carried out, patients showed an increase in pulse wave amplitude, a reduction in ischaemic pain, and in the frequency and severity of RP attacks, together with an increase in DU healing which, in severe cases, prevented digital amputation, the use of opioid analgesics and hospitalisation.11,12,22,23 It also improved skin colour, temperature, sensitivity and mobility (due to reduced oedema in the hands).11,12 GT has been shown to improve cutaneous sclerosis also in patients with morphea,24 and post-GT improvement of digestive symptoms, such as mouth opening, mild oesophagitis and oesophageal hypomotility, described in patients with SSc.25
It is a safe and well-tolerated treatment in patients of a wide age range, with no significant side effects under the conditions applied. The hypergravity protocol is adjusted (number of sessions, maximum g level reached) according to the patient's tolerance, age and clinical characteristics.6 Transient dizziness or nausea may occur when the patient is not yet accustomed to the procedure, or occasionally vomiting/dizziness related to head movement during centrifugation (Coriolis effects),6 Patient training is important for better tolerance and continuity of treatment.
Given the extensive experience in Uruguay with GT and its efficacy in SSc, with an excellent safety profile, availability and low cost, added to the fact that it is a therapeutic option that is not widely known in the scientific community, this research we are conducting could provide future scientific, health and economic benefits.
ObjectiveTo assess the efficacy of GT in skin and vascular conditions, in quality of life and functional capacity, and its safety profile in patients with SSc.
MethodologyStudy designDescriptive, retrospective study of a cohort of patients with SSc treated at the Systemic Autoimmune Disease Unit (UEAS for its initials in Spanish) of the Military Hospital, a reference centre for autoimmunity in Montevideo, Uruguay.
Patients and variablesInclusion criteria: patients over 18 years of age, with a confirmed diagnosis of SSc (ACR/EULAR 2013 criteria),26 treated with GT (minimum 10 sessions) during the period April 2013-April 2023.
Exclusion criteria: no consent to participate, overlap with other systemic autoimmune diseases (SAD). Data were obtained from the review of the patients' medical records. The variables of cutaneous and vascular efficacy, quality of life and functional capacity were compared with each other, before and after GT.
For cutaneous efficacy assessment, the following were recorded: the modified Rodnan score (mRSS) in 17 areas27 and its pre/post-GT variation. The initial mRSS (pre-GT) was considered to be the one recorded in session 0 of the GT and the final mRSS (post-GT) was considered to be the one corresponding to the highest number of sessions with respect to session 0. The correlation between the variation in the pre/post-GT mRSS and the cutaneous extension, the immunological profile and the years of evolution of SSc (at the beginning of the GT) was explored. The presence/absence/improvement of puffy fingers, sclerodactyly, pitting scars and telangiectasias was also recorded. Patients without complete pre- and/or post-GT mRSS data and with SSc without scleroderma were excluded from the cutaneous efficacy analysis. The cutaneous extension of SSc was classified as limited (lSSc), diffuse (dSSc) or SSc without scleroderma according to Le Roy criteria.28
Antibody (AB) profile: determination of antinucleocytoplasmic AB (ANA) by indirect immunofluorescence on Hep-2 cells (titres greater than 1/80 were considered positive).29 In the case of positive ANA, specific AB were determined by ELISA technique: anti-DNA, anti-neutrophil cytoplasm (ANCA), and by immunoblot: anti-RNP/SM, anti-DNA, anti-Smith, anti-ribonucleoproteins (anti-RNP), anti-Ro 60 (SS-A), anti-Ro 52 (SS-A), anti-La, anti-Jo1, and those specific for SS, anti-topoisomerase I (anti-Scl 70) and anti-centromere. Other AB specific for SS30 and those associated with overlap syndrome with scleroderma30 are not available from the health provider. The "other AB of interest" category included antiphospholipids (anticardiolipin, anti-beta 2-glycoprotein 1 by ELISA technique and lupus anticoagulant by coagulometric techniques), anti-citrullinated peptide, rheumatoid factor and ANCA.
For the vascular efficacy assessment, the following were considered: the severity of the RP (according to the Taylor-Pelmear scale),31 the number of DUs, necrotic lesions and amputations, as well as vascular pain, measured by a visual analogue scale (VAS) from 0 to 10 performed by the patients.
For quality of life and functional capacity assessment, the Cochin Hand Function Score (CHFS)32 and the Mouth Handicap in Systemic Sclerosis (MHISS)33 scales validated in SSc were used. To complement this assessment a self-administered and anonymous questionnaire (GT questionnaire) was developed, with results reported by patients (RRP) on the benefits of GT. It also included questions on tolerance to treatment and adherence to it (Appendix A Supplementary I).
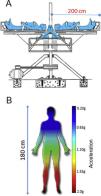
Gravitational therapyGT was performed by specialists from the Gravitational Therapy Centre (GTC) in Montevideo. Patients were exposed to acceleration/deceleration (+Gz) profiles (gradient of g levels from head to toe) from 0 to 1.5–2.5 g at the foot level (Fig. 1) with a rapid onset to peak acceleration and a rapid deceleration afterwards. The GT protocol was adjusted according to each patient's tolerance. The average duration of the protocol was 1 h, 1–3 times/week, until completing 20 sessions. In some cases, maintenance therapy was performed (10–20 sessions per year), depending on their clinical situation. All patients maintained their usual pharmacological treatment.
Drawing of the human centrifuge at the Gravitational Therapy Centre and + Gz gradients. (A) Centrifuge of almost 2 metres in diameter used for GT sessions at the Gravitational Therapy Centre in Montevideo, Uruguay. Maximum 4 patients in one session. (B) Example of a gravity gradient on a 180 cm tall patient exposed to a maximum of 2 g at foot level in a 2 m diameter system. Adapted from: Isasi et al., 2022, doi: 10.3389/fphys.2022.952723.
In the quantitative variables, the means were compared with the t-test, once the homogeneity of variance and normality of the data had been tested. The McNemar test was applied to identify whether there are differences between the proportions of the paired samples. Frequency distributions (number and percentage of total patients) were used for qualitative variables. The software Rstudio (version 2022.07.2) and R for Windows (version R-4.2.1) were used.
ResultsA total of 50 patients were included. Women predominated (96%), Caucasian (98%), and the mean age of the cohort was 62 ± 12 years. Table 1 contains the demographic characteristics, the SSc status at the start of GT and at the time of inclusion in the study, the cutaneous extension and the immunological profile of the patients.
Demographic characteristics, time of evolution, cutaneous extension and immunological profile of patients with systemic sclerosis included in the study.
| Characteristics | Number of patients (%) |
|---|---|
| Total | 50 |
| Gender (women/men) | 24/1 (96/4) |
| Age at study (years) | 62 ± 12 |
| Race | |
| Caucasian | 49 (98) |
| Afro-descendants | 1 (2) |
| SSc duration at inclusion in the study (years) | 6.8 ± 3.2 |
| SSc duration at the beginning of GT (years) | 2.8 ± 3.2 |
| Immunological profile: (n = 43) | |
| ANA positive groupa | 10 (20) |
| Anti centromere positive group | 15 (30) |
| Scl70 positive group | 11 (22) |
| ANA negative group | 7 (14) |
| Cutaneous extension (n = 49) | |
| Diffuse (dSSc) | 20 (40) |
| Limited (lSSc) | 26 (52) |
| No scleroderma (sine) | 3 (6) |
Qualitative variables are expressed as number and/or percentage, and quantitative variables, with mean ± standard deviation.
SSc: systemic sclerosis.
ANA positive group includes: ANA positive, anti-centromere and anti-Scl70 negative patients. ANA negative group includes: ANA, anti-centromere and anti-Scl70 negative patients. Anti-centromere positive patients associated: lSSc (11), dSSc (2), sine (2). Scl70 positive patients associated: lSSc (3), dSSc (7), none sine. Scl70 and anti-centromere negative patients associated: lSSc (7), dSSc (7), sine (1). Scl70 and anti-centromere positive patients associated: lSSc (2). 8 patients excluded from the analysis due to incomplete data.
No significant adverse effects were recorded during the treatment.
Analysis of cutaneous efficacyFor this analysis, 3 patients without scleroderma and 22 without pre- and/or post-GT data were excluded. A total of 25 patients were analysed. Post-GT, a significant improvement in mRSS was observed (pre-GT 19.2 ± 8.7 vs. post-GT 5.4 ± 5.0 points, t-test, p < .05), average mRSS reduction post vs. pre-GT: 13.8 ± 5.7 points (Table 2, Fig. 2). 75% of patients improved mRSS by 11 points or more. The mean time to complete 20 GT sessions was 137.2 ± 99.3 days. The mean reduction in mRSS in patients who received 10 sessions was 8.8 points vs. 13.1 in those who received 20 sessions. The reduction in mRSS post-GT was greater in patients with higher initial mRSS (R = .84, p < .05) (Fig. 3). The value of the variation in mRSS pre/post-GT was not significantly related to the time of evolution of SSc at the beginning of GT (<1 year, 1−4 years, >4 years), with the immunological profile of the patients or with the cutaneous extension of SSc. In the RRP, 36/38 (95%) of the patients who completed the questionnaire reported improvement at the cutaneous level, 87% responded that it improved quite a bit or a lot and 8% that it improved little. On the other hand, a significant improvement was observed in the number of patients with puffy fingers (pre-GT 25/50 [50%] vs. post-GT 10/50 [20%], McNemar test, p < .05), but not in the frequency of patients with telangiectasias, pitting scars or sclerodactyly (Table 3).
Modified Rodnan score (mRSS) pre and post gravity therapy and its variation.
| n | Q25 | mean | Q75 | SD | min | max | |
|---|---|---|---|---|---|---|---|
| Pre-GT | 25 | 15 | 19.2 | 24 | 8.7 | 2 | 37 |
| Post-GT | 25 | 2 | 5.4 | 8 | 5.0 | 0 | 19 |
| Variation | 25 | 11 | 13.8 | 17 | 5.7 | 0 | 23 |
A significant decrease in mRSS is observed, pre-GT 19.2 ± 8.7 and post-GT 5.4 ± 5.0 (t-test, p < .05). The average reduction in mRSS post- vs. pre- GT was 13.8 ± 5.7 points.
Comparison between the pre-treatment modified Rodnan score (mRSS) and its change. A significant correlation is observed between the magnitude of the reduction in mRSS (post-GT - pre-GT) and the initial pre-GT value (R = .84, p < .05). The reduction in mRSS was greater in patients with higher initial mRSS.
Effects of gravity therapy on puffy fingers, sclerodactyly, pitting scars and telangiectasias.
| Clinical symptom | Time of registration | Number of patients (%) |
|---|---|---|
| Puffy fingers | BGT | 25 (50) |
| AGT | 10 (20) | |
| Sclerodactyly | BGT | 36 (72) |
| AGT | 33 (66) | |
| Pitting scars | BGT | 5 (10) |
| AGT | 2 (4) | |
| Telangiectasias | BGT | 15 (30) |
| AGT | 10 (24) |
A significant improvement was observed in the number of patients with puffy fingers (pre-GT 50% vs. post-GT 20%, McNemar test, p < .05), but not in telangiectasias, pitting scars or sclerodactyly.
BGT: before gravity therapy; AGT: after gravity therapy; n = 50 patients.
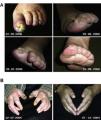
A significant decrease in the severity of RP was observed in most patients, the majority with severe RP at baseline: pre-GT grade 3–4 RP, 43/48 (89.6%) patients vs. post-GT, grade ≤2, 42/47 (89.4%) patients. In total, 38/48 (81%) of the patients improved the grade of RP, while 9 did not register changes. Therefore, the probability of having severe RP grade 3–4 decreases post-GT (McNemar test, p < .05) (Table 4). From the patient's perspective, 32/35 (91%) of the patients reported improvement in RP, 74% responded that it improved quite a bit or a lot and 17% that it improved little. On the other hand, a notable improvement was observed in vascular pain quantified by VAS (pre-GT, 7.6 ± 2.2, post-GT, 1.4 ± 1.2, p < .05). The RRPs report significant improvement in vascular pain and also in joint/muscle pain (Table 5). Regarding the DUs, pre-GT, 7 patients had 15 fingers with critical ischemia, post-GT, 5 achieved complete healing of fingers and 2 patients partial healing (1 amputation each). In total, there was improvement of DU in 11 fingers (Table 4, Fig. 4).
Effect of gravitational therapy in the Reynaud phenomenon, digital ulcers and vascular pain.
| Number of patients (%) | ||
|---|---|---|
| Severity of RP | BGT | G 0: 1 (2)G 1: 1 (2)G 2: 3 (6.2)G 3: 35 (72.9)G 4: 8 (16.7) |
| AGT | G 0: 13 (27.7)G 1: 21 (44.7)G 2: 8 (17)G 3: 0G 4: 1 (2.1) | |
| Digital ulcers | BGT | 7 (14.5) |
| AGT | 0 | |
| Mean ± SD | ||
| Vascular pain by VAS | BGT | 7.6 ± 2.2 |
| AGT | 1.4 ± 1.2 |
Quantitative variables are expressed as mean ± standard deviation, and qualitative variables as number (percentage).
BGT: before gravity therapy; AGT: after gravity therapy; VAS: visual analogue scale. 2 patients without BGT data and 7 patients without AGT data; RP: Raynaud's phenomenon.
A significant decrease in the severity of RF was observed in the majority of patients (most with severe RF at baseline): pre-GT grade 3–4, 43 (89.6%) of patients vs. post-GT, 46 (89.4%) grade ≤2, McNemar test, p < .05), as well as vascular pain measured by VAS: pre-GT, 7.6 ± 2.2, post-GT, 1.4 ± 1.2, p < .05).
Results reported by the patients in a gravitational therapy questionnaire.
| Variable | Improvement (% of patients) |
|---|---|
| Quality of life | 97 |
| Reynaud’s phenomenon | 89.5 |
| Skin | 95 |
| Capacity for ADL | 89.5 |
| Mood | 65 |
| Sleep disorders | 37 |
| Other variables | % patients |
|---|---|
| Vascular pain (VAS > 4), pre-GT | 65 |
| Vascular pain (VAS ≤ 4), post-GT | 84a |
| Muscular/joint pain (VAS > 4), pre-GT | 60 |
| Muscular/joint pain (VAS ≤ 4), post-GT | 74a |
| Tolerance to “good” GT | 100% |
| 100% adherence to sessions | 87% |
ADL: activities of daily living.
The results correspond to questionnaires completed by 38 patients.
Effect of gravity therapy on digital vascular lesions in patients with systemic sclerosis. (A) Images of the effect of gravity on a digital ulcer of a left lower limb at baseline and its evolution with gravity in a patient with SSc. (B) Evolution of digital necrosis in a patient with mixed connective tissue disease at baseline (left) and post-GT (right). These images do not correspond to patients in this study. Adapted from: Isasi et al., 2022, doi: 10.3389/fphys.2022.952723. Photos courtesy of the Gravitational Therapy Centre.
A decrease in CHFS and MHISS was recorded (mean value pre-GT: 10.1 and 9.9, respectively (21 patients), and mean value post-GT: 7.9 and 8.1, respectively (9 patients). The RRPs show that 34/38 (89%) of patients report improvement in the ability to perform activities of daily living and 37/38 (97%) of patients report improvement in quality of life. Table 5 summarizes the RRPs of 38 patients who completed the GT questionnaire.
Discussion and conclusionsGT significantly improved skin involvement in patients with SSc, as previously demonstrated in previous studies by Isasi et al.11,12,23 The more sessions performed the greater the benefit, which is evident from the comparison of the decrease in mRSS depending on whether 10 or 20 sessions are performed. However, as previously published,11,12 when improvement appears, it is early, and is already observed in the first 10 sessions. The time in which the results were achieved (20 sessions) was relatively short (just over 4 months). Although long-term follow-up of patients was not an objective of this study, the maintenance over time of the improvement in mRSS post-GT is variable, as evidenced by the review of clinical records and according to the opinion of CGT experts. The indication for annual maintenance GT should therefore be individualised. Patients with the highest initial mRSS were those who showed the greatest reduction in mRSS post-GT, probably due to their greater capacity for improvement due to more extensive skin involvement, and at the expense of improving skin areas with less fibrotic involvement. The initial mRSS and its post-GT variation were not related to the time of evolution of SSc at the beginning of GT, nor to the immunological profile of the patients or the cutaneous extension of the disease. This should be interpreted with caution, since the number of patients with mRSS presented in this study is small, there are no previous complete investigations of SSc patients treated with GT that allow comparing the results of this study with other cohorts, and a control group of patients (without GT) was not included in this analysis.
A significant improvement was recorded in the number of patients with puffy fingers, with the decrease in hand oedema being a fact previously reported by Isasi et al.12 However, GT did not influence the frequency of patients with sclerodactyly, pitting scars or telangiectasias.
At the vascular level, the severity of RP decreased significantly after GT in most patients. Moreover, there was a cure of DU, which prevented hospitalisation and eventual digital amputation, and the use of vasodilators such as intravenous prostacyclins, not available in Uruguay, was not necessary. Patients significantly improved ischaemic pain, which decreased the use of opioid analgesics. These results are similar to those previously reported.11,12 Functional capacity measured by CHFS and MHISS improved, although the number of patients with these data was small.
In the RRP, most patients reported improvement in skin, RP, ischaemic pain and joint/muscle pain (statistically significant improvement), and other data emerge, such as improvement in mood and sleep disorders, reported by a smaller number of patients. All these benefits necessarily impact on quality of life and functional capacity, and this was stated by the majority of patients in the GT questionnaire. In this study, no significant adverse effects were recorded, similar to that observed in the GTC, even with patient follow-ups for more than 20 years. This is also reflected in the GT questionnaire, in which all patients expressed good tolerance to the treatment and high adherence to it.
Although this study has limitations given its descriptive and retrospective design and the absence of a control group of patients with SSc, not treated with GT, the results are highly promising. The effect of drug treatment on the results was not analysed, which could be a starting point for future research.
This research study contributes to the dissemination of GT and its results, which coincide with the benefits observed in daily clinical practice and with those reported by patients. Controlled and randomised clinical trials are needed, to compare the effects of GT vs. conventional drug treatment. In addition, more basic research is needed to unravel the biological mechanisms and the cellular and molecular bases of clinical improvement, as well as for its international validation and widespread use.
ConclusionsGT improved cutaneous and vascular involvement, quality of life and functional capacity in patients with SSc with an excellent safety profile. Randomised controlled clinical trials are needed to corroborate these observational results.
CRediT authorship contribution statementL.F.S., E.I., M.E.I.: conception and design of the work, data collection, analysis and interpretation of data and results, statistical analysis, writing of the manuscript, critical revision of the manuscript. AP, S.P.: data collection.
FundingThis research did not receive any specific support from public sector agencies, commercial sector or non-profit entities.