To analyse the efficacy and safety of intra-articular injection of expanded Mesenchymal Stromal Cells (MSCs) in knee osteoarthritis.
MethodsSystematic Literature Review. A pre-defined search strategy was run in Medline, Embase and Cochrane Library until February 2018. Inclusion criteria: knee osteoarthritis (grades II-IV Kellgren-Lawrence); intra-articular injection of MSCs (without surgical co-treatments); Randomized Controlled Trials (RCTs) or Quasi-experimental Clinical Trials (QCTs) N ≥ 10 and ≥6 months of follow-up were included. Evidence was assigned according to the Scottish Intercollegiate Guidelines Network (SIGN).
ResultsThe search identified 252 articles. Nine proof-of-concept trials (3 RCTs, 6 QCTs) were included (N = 169). Evidence showed clinical improvement in 60% of patients. Structural benefit was reported in half of patients. Clinical benefit was observed from the 3rd month and structural improvement from the 6th. All studies reported maximum clinical and structural benefit a year following the implant. This benefit was sustained for up to 24 months. Studies with doses ≥40 × 106 showed more consistent clinical and structural benefits than those with lower doses. No systemic adverse reactions were reported. The most common adverse effect was pain and/or inflammation in the puncture area (13–53%). The use of donor cells was as safe as autologous implants.
ConclusionsIntra-articular implants of MSCs seem to be safe with no serious adverse effects. Low-quality evidence precludes conclusions regarding efficacy in this review. However, the clinical and structural benefits observed provide a rationale for using expanded MSCs implants in osteoarthritis patients. High-quality evidence trials are needed to further determine best protocols to maximize clinical and structural improvement.
Analizar la eficacia y la seguridad de los implantes de células mesenquimales estromales expandidas (MSCs, por sus siglas en inglés) en la artrosis de rodilla.
MétodosRevisión sistemática de la literatura. Estrategia de búsqueda en Medline, Embase y Cochrane Library hasta febrero del 2018. Inclusión: artrosis de rodilla (II-IV Kellgren); inyección intra-articular de MSCs; ensayos controlados aleatorizados (ECA) y ensayos clínicos cuasi-experimentales (ECC) ≥ 6 meses y N ≥ 10.
ResultadosDe 252 artículos identificados, se incluyeron 3 ECA y 6 ECC (N = 169). El 60% de los pacientes mejoraron clínicamente y el 50% estructuralmente hasta 2 años después del implante. El beneficio máximo se alcanzó al año con dosis ≥40 × 106 MSCs. La intervención fue bien tolerada, igualmente segura en implantes alogénicos y autólogos.
ConclusionesLas MSCs intra-articulares son seguras. La baja calidad de la evidencia analizada no admite conclusiones sobre la eficacia, pero prueba un fundamento para su uso en la artrosis como modificador de síntoma y de estructura que debe ser constatado en ensayos de alta calidad.
Advanced cellular therapies are one of the most cutting edge fields in the treatment of osteoarthritis. These are seemingly safe treatments which prospectively fulfil the dual notion of modifying symptom and structure. The greatest protagonist stems from the adult progenitor cells, since they have greater biologic plasticity than the differentiated adult cells (chondrocytes) and are safer than the embryonic progenitor cells. Mesenchymal Stromal Cells [MSCs] are adult progenitor cells derived from the embryonic mesoderm with a marked capacity for self-renewal and depending on the microenvironment in which they are found, adult cells of different mesenchymal lineages may be differentiated (chondrocyte, osteoblast, aipocyte, myocyte and tenocyte).1 This double condition (self-renewal and differentiation) makes them extraordinarily attractive for regenerative strategies of mesenchymal tissues, such as cartilage or bone.2 In animal models of osteoarthritis, MSCs have reproducibly shown their marked ability to repair chondral damage, detain progression and improve symptoms.3 However, clinical experiences with MSCs have not been able to reproduce these virtues, offering a panorama of mixed, inconsistent results.4,5 Part of this discrepancy may be explained by the notable terminological confusion surrounding the treatment with stem cells. Rather than a single therapy, this involves several very different cellular treatments.6 The most commonly used in clinical practice are characterized by minimal tissue manipulation, marked cellular heterogeneity, a great wealth of growth factors and, above all, a marginal proportion of MSCs.7,8 In these treatments, it is highly complex to discriminate the therapeutic role of the MSCs due to the large quantity of therapeutic agents (potentially) involved.
In order to determine the efficacy and safety of MSCs in osteoarthritis, we did a systematic review of the literature (SRL) which evaluated the treatment of osteoarthritis knees with intra-articular implants of expanded MSC, without scaffolds or surgical co-treatments.
Material and methodsSearch strategyA sensitive search was performed in Medline, Cochrane Library and Embassy (up to February 2018) designed by an expert documentalist (MA), using Mesh and free text terms in English and Spanish (Table 1). The aim was to identify relevant publications which could have been overlooked. The electronic search was complemented with a manual search into the references of the selected studies.
Search strategy.
| Pubmed Results of the search: 168 |
|---|
| (“Osteoarthritis, Knee”[Mesh]) AND (((((((((((“Stromal Cells”[Mesh]) OR “Multipotent Stem Cells”[Mesh]) OR “Mesenchymal Stromal Cells”[Mesh]) OR “Stem Cells”[Mesh]) OR “Fetal Stem Cells”[Mesh]) OR “Pluripotent Stem Cells”[Mesh]) OR “Embryonic Stem Cells”[Mesh]) OR “Induced Pluripotent Stem Cells”[Mesh])) OR ((“Stromal Cells”[Title/Abstract] OR “Multipotent Stem Cells”[Title/Abstract] OR “Mesenchymal Cells”[Title/Abstract] OR “stem cell”[Title/Abstract] OR “embryonic”[Title/Abstract] OR “Induced Pluripotent Stem Cells” OR))) OR ((“Progenitor Cell* Mesenchymal”[Title/Abstract] OR “Mother Cell*”[Title/Abstract] OR “Colony-Forming Unit*”[Title/Abstract] OR “IPS Cell*”[Title/Abstract]))). |
| Filters: Spanish, English, humans. |
| Embase Results of the search: 25 |
| (knee NEAR/3 osteoarthritis) AND ((‘stromal cell’/de OR 'multipotent stem cell'/de OR 'stem cell'/de OR 'fetal stem cell'/de OR 'pluripotent stem cell'/de OR 'embryonic stem cell'/de OR 'induced pluripotent stem cell') OR ('progenitor cell mesenchymal’ OR 'mother cell' OR 'colony forming unit')) AND [embase]/lim NOT ([embase]/lim AND [medline]/lim) AND ([English]/lima OR [Spanish]/lim) AND [humans]/lim. |
| Filers: Spanish, English, humans. |
| Cochrane Results of the search: 59 |
| [mh “Osteoarthritis Knee”] OR (knee near/3 osteoarthritis) AND ([mh “Stromal Cells”] or [mh “Multipotent Stem Cells”] or [mh “Mesenchymal Stromal Cells”] or [mh “Stem Cells”] or [mh “Fetal Stem Cells”] or [mh “Pluripotent Stem Cells”] or [mh “Embryonic Stem Cells”] or [mh “Induced Pluripotent Stem Cells”] OR (“Stromal Cells” or “Multipotent Stem Cells” or “Mesenchymal Cells” or “stem cell” or “embryonic” or “Induced Pluripotent Stem Cells”) OR ('stromal cells' or 'multipotent stem cells' or 'mesenchymal cells' or 'stem cell' or 'embryonic' or 'induced pluripotent stem cells') OR ('progenitor cell mesenchymal' or 'mother cell' or 'colony forming unit')). |
| Filters: Spanish, English, humans. |
Study selection was made jointly by 2 reviewers (PA) and (JM) in 2 phases: 1) selection by titles and abstracts; 2) recompilation of the complete text with selected references. The said references were analysed by looking at previously established inclusion criteria: adult patients (N ≥ 10), with moderate-severe osteoarthritis of the knee (grade II-IV Kellgren-Lawrence), treated with at least one intra-articular injection of MSCs (according to the phenotype criteria of the ISCT)9 and with minimum follow-up of 6 months. Exclusively level I studies were considered for inclusion: meta-analyses, systematic reviews of prospective studies, randomised clinical trials with control group (randomised controlled trials ) [RCT]) and with no control group (quasi-experimental clinical trials) [QCT]). The following were excluded: observational studies and intra-articular interventions with cellular populations which did not meet with ISCT criteria. Equally, those trials where treatment was administered together with co-interventions with scaffolds or surgical procedures were excluded.
Data collection and assessment of qualityData collection of selected publications was carried out by one of the reviewers (PA) with the use of specific, pre-designed templates. When doubt ensued for inclusion of a study, a second critical reading was made by the second reviewer (JM). The final inclusion was determined by consensus. Final data collected in templates were used for the creation of the final summary of findings table.
The quality of the evidence was ranked according to the scale of the Scottish Intercollegiate Guidelines Network (SIGN).10 The SIGN method places particular emphasis on quantitative analysis provided by the SRL and also places importance on the reduction of systematic error or bias.
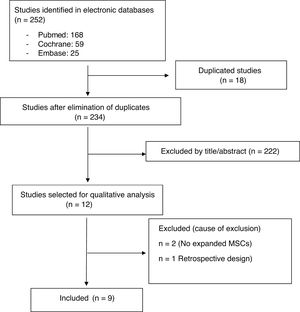
ResultsStudy descriptionThe electronic search identified 252 references (18 duplicates were ruled out). After Reading the title and abstract 222 studies were excluded. Twelve relevant articles were selected for critical reading (Fig. 1). Nine met with the inclusion critieria11–19 and 3 were excluded (2 made with cellular implants which did not meet with ISCT20,21 criteria and one observational study22). The body of evidence consisted of 9 clinical trials where 169 patients were treated with MSCs (61.8% women and 38.1% men). All clinical trials were concept tests (Phase I-II), 3 RCT controlled with hyaluronic acid (HA)12,14,19 and 6 QCT.11,13,15–18 No clinical efficacy trial (Phase III) met with inclusion criteria. One hundred and fifteen patients were treated with MSCs obtained from bone marrow11,12,14,15,17,19 and 54 with MSCs from adipose tissue.13,16,18 Two studies used more than one dose of MSCs11,18 and 2 used allergenic MSCs implants.12,19 Follow-up times ranged between 6 and 24 months. The quality of the evidence assigned was the lowest of that contemplated in the SIGN scale for clinical trials (SIGN 1−), due to the low sample number of studies and that the majority of trials had no control group (Table 2).
Summary of evidence
| Author, year of publication (reference) | Type of study and follow-up | Population (number, percentage of women, mean age, eligibility) | Intervention | Measurements of outcome | Results | SIGN Quality |
|---|---|---|---|---|---|---|
| Gupta et al., 201612 | RCT 12 m | N = 6050%56 ± 6 yearsKellgren II-III; pain which requires NSAID | allogenic BM MSCs (Stempeucel©)25 × 106 (2 ml) + HA n = 10; Pl + HA n = 550 × 106 (2 ml) + HA n = 10; Pl + HA n = 575 × 106 (4 ml) + HA n = 10; Pl + HA n = 5150 × 106 (4 ml) + HA n = 10; Pl + HA n = 5HA = Hyalgan® 2 ml | Safety: PE, EKG, haemogram, ESR, BB at 1, 12, 24 and 48 wEfficacy: VAS, WOMAC, ICOAP at 1, 12, 24 and 48 wStructural NMR (0, 24 and 48 w); KX (0, 12, 24 w) | Safety: safe at 12 m. 16% post-puncture discomfort. One hospitalisation due to synovial fluid Efficacy: No improvement Structural No improvement | Quality 1−Comments: low sample in asymmetrical groups. Low discriminatory power. Short follow-up for structural efficacy; heterogeneous intervention |
| Lamo-Espinosa et al., 201614 | RCT 12 m | N = 3037%61,3 ± 5 yearsKellgren II-IV; VAS ≥ 2,5; BMI 20-35 | Autologous BM MSCs Low dose: 10 × 106 (1,5 ml) + HA n = 10High dose: 150 × 106 (3,5 ml) + HA n = 10Control: HA n = 10HA = Hyalone® 4 ml | Safety: PE, haemogram, BBEfficacy: VAS, WOMAC 0, 3, 6, 12 m“WOMAC responders” = ↓20% 2 items and >↓10 points of total scale Structural NMR and KX (0, 6 and 12 m) | Safety: safe at 12 m. 13% post-puncture discomfortEfficacy: ↓ VAS from 3 m, maximum 12 m. No differences between groups; “WOMAC responders” with High doses at 12 mStructural improvement at 12 months only in high doses (4 WORMS points) | Quality 1−Comments: low sample; no masking; heterogeneous groups; low discriminatory power. Short follow-up for structural efficacy |
| Vega et al., 201519 | RCT 12 m | N = 3057%57 ± 9 yearsKellgren II-IV; Chronic refractory pain ≥ 6 m | Allogenic BM MSCs MSCs: 40 × 106 (1,5 ml) n = 15Control: HA (Durolane® 3 ml) n = 15Source MSCs: 3 healthy donors from the sample | Safety: PE, haemogram, BBEfficacy: VAS, WOMAC, Lequesme (0, 3, 6, 12 m)Structural NMR (0, 6 and 12 m) | Safety: safe at 12 m. 53% post-puncture discomfortClinical Efficacy: significant improvement with VAS, WOMAC, Lequesme from 6 mStructural improvement at 12 months of PCI | Quality 1−Comments: low sample; no masking; low discriminatory power. Short follow-up for structural efficacy |
| Jo et al., 201713 | QCT 6 m | N = 1883%61 ± 6 yearsKellgren III-IV; VAS 4 ≥ 4 m | Autologous AT MSCs Low dose: 10 × 106 (3 ml) (n = 3)Medium dose: 50 × 106 (3 ml) (n = 3)High dose: 100 × 106 (3 ml) (n = 12) | Safety: PE, haemogram, BBEfficacy: VAS, WOMAC, KSS, KOOS 0, 1, 2, 3, 6, 12, 24 mStructural NMR (3, 6 and 24 m) | Safety: safe at 24 mClinical Efficacy: significant improvement only with high doses: VAS, WOMAC, KOOS and KSS (maximum at 12 m)Structural improvement with High doses at 6 m of interline, ↓ chondral defects and ↑ cartilage column. Then plateau | Quality 1−Comments: low sample; lost data; no control; safety data incomplete; arthroscopy bias |
| Pers et al., 201616 | QCT 6 m | N = 1856%64 ± 8 yearsKellgren III-IV; Chronic pain ≥ 12 m | Autologous AT MSCs (from VSF)Low dose: 2 × 106 (5 ml) (n = 6)Medium dose: 10 × 106 (5 ml) (n = 6)High dose: 50 × 106 (5 ml) (n = 6) | Safety: PE, haemogram, BB.Efficacy: VAS, WOMAC, PGA, SAS, SF-36, KOOS 0, 1 s, 3 m, 6 m“Responder” = ↓20% VAS and WOMACStructural NMR (0, 4 m) | Safety: safe 22% post-puncture discomfortClinical efficacy: Significant improvement from 1.ª only with Low doses: VAS, WOMAC, KOOS and responders (maximum at 3 m)Structural improvement inconclusive | Quality 1−Comments: low sample; lost data; no control; heterogeneous groups; NMR results incomplete |
| Song et al., 201618 | QCT 24 m | N = 1878%55 ± 10 yearsKellgren II-III; VAS ≥ 4 > 4 m | Autologous AT MSCs PHASE I (2 doses separated by 3 w)Low dose: 10 × 106 (3 ml) (n = 6)Medium dose: 20 × 106 (3 ml) (n = 6)High dose: 50 × 106 (3 ml) (n = 6)PHASE IIa (additional dose 50 × 106 after one year) | Safety: PE, EKG, haemogram, ESR, BB every 2 s × 12 mEfficacy: WOMAC, NRS-11, SF-36 (0, 12, 24, 48, 72, 96 w)Structural NMR (0, 12, 24, 48, 72, 96 w) | Safety: safe 44% post-puncture discomfortClinical efficacy: consistent only with high doses. NRS-11 improvement from 3-24 m; WOMAC from 6 to 12 m, then plateauStructural increase thickness of cartilage greater with high doses | Quality 1−Comments: potential multi-dose benefit; low sample; no control; results biased for a efficacy; heterogeneous groups; confusing methodology |
| Al-Najar et al., 201711 | QCT 24 m | N = 1378%50 ± 10 yearsKellgren II-III; Chronic pain ≥ 6 m | Autologous AT MSCs Two doses 30.5 × 106 (5 ml) separated by 4 w | Safety: PE (1, 2, 4, 12 s and then every 6 m), haemogram and BB (3, 24 m)Efficacy: KOOS (1, 2, 4, 6, 12, 24 m)Structural NMR (6, 12 m) | Safety: safe 23% post-puncture discomfortClinical efficacy: improvement KOSS from 6 to 12 m, then plateauStructural increase of cartilage thickness at 12 m | Quality 1−Comments: very low sample and biased for efficacy; no control group |
| Orozco et al., 201315 | QCT 12 m | N = 1250%49 ± 5 yearsKellgren II-IV; Refractory pain ≥ 6 m | Autologous BM MSCs Single dose 40 × 106 (8 ml) | Safety: PE, haemogram, BB (1 s, 3, 6, 12 m)Efficacy: VAS (0, 3, 6, 12 m), WOMAC and Lequesme (0 y 12 m)Structural NMR (. 6 and 12 m) | Safety: safe at 12 m. 50% post-puncture discomfortClinical efficacy: significant VAS improvement from 3 m, maximum at 12 m. Improvement WOMAC, Lequesme at 12 mStructural improvement at 6 and 12 m of PCI | Quality 1−Comments: improvement and safety maintained in follow-up at 24 m.Very low sample and biased for efficacy; no control |
| Soler et al., 201517 | QCT 12 m | N = 1560%52 ± 5 yearsKellgren II-III; Chronic mechanical pain | Autologous BM MSCs Single dose: 40.9 × 106 ± .4 × 106 (10.0 ± .3 ml) | Safety: PE, haemogram, BB (1 w, 3, 6, 12 m)Efficacy: VAS (0, 3, 6, 12 m), HAQ pain, SF-36, WOMAC and Lequesme (0, 6 and 12 m)Structural NMR, T2 mapping of composition of collagen of the cartilaginous matrix (. 6 and 12 m) | Safety: safe at 12 m. 60% post-puncture discomfort. Lumbago 27%Clinical efficacy: significant VAS improvement from 8 d, maximum to 12 m. Improvement WOMAC, Lequesme at 12 m. No improvement SF-36Structural significant reduction of T2 map at 12 months | Quality 1−Comments: improvement and safety maintained at 4 years. Better in patient candidates with moderate osteoarthritis. Very low sample and biased for efficacy; no control group |
AT: adipose tissue; BB: blood biochemistry; BM: bone marrow; BMI: body mass index; EKG: electrocardiogram; HA: hyaluronic acid; HAQ: Health Assessment Questionnaire; ICOAP: Intermittent & Constant Osteoarthritis pain score; KOOS: Knee injury and Osteoarthritis Outcome Score; KSS: Knee Society clinical rating system; KX: knee X-ray; NMR: nuclear magnetic resonance; NSAID: non steroid anti inflammatory drugs; PE: physical examination; PCI: poor cartilage index; PGA: Patient Global Assessment; Pl: placebo; QCT: quasi-experimental clinical trials; RCT: random controlled trials; SAS: Short Arthritis Assessment Scale; SF-36: Short Form questionnaire; SIGN: Scottish Intercollegiate Guidelines Network; VSF: vascular stromal fraction; VAS: Visual Analogue Scale; ESR: erythrocyte sedimentation rate; W: week; WOMAC: Western Ontario & McMaster Universities Osteoarthritis Index; WORMS: Whole-Organ Magnetic Resonance Imaging Score.
Efficacy of intra-articular treatment with MSCs was assessed clinically and structurally.
Clinical efficacyTwo controlled studies reported significant improvement regarding hyaluronic acid.14,19 Lamo-Espinosa et al.14 found there was an analgesic improvement (VAS) with all doses used. This improvement was progressive from the third month until the maximum of one year. The WOMAC improvement was only observed in patients treated with high doses and was later (one year) (Table 2). In the other RCT, the magnitude of the analgesic and functional improvement was greater than that of the HA from 6 m onwards and it also reached its maximum at one year.19 The third RCT included was adjusted to the same time curve with a maximum response at one year, but with no significant differences regarding HA.12
All QCT reported clinical improvement with respect to their baseline situation (Table 2). In several studies the analgesic benefit (VAS and WOMAC pain) was observed from 3 months onwards.15–17 However, the improvement of the other functional measurements (not just analgesic ones) did not occur until 6 months. Like with the RCT, the maximum peak clinical benefit was reported one year after implant. This response was maintained in some patients until 24 months.11,13,18 In general, the clinical response was more consistent in patients treated with the highest doses.13,16,18
Structural efficacyThe structural modifying effect of the implant with MSCs was reported in 2 RCT14,19 and 5 QCT.11,13,15,17,18 In the RCT, the structural benefit was significant with regard to the HA from one year onwards. After 12 months of implant, patients with MSCs showed a significant reduction in joint space,14 in the Whole-Organ Magnetic Resonance Imaging Score (WORMS)14 and the low quality cartilage area (PCI)19 (Table 2). This structurally modifying effect was only observed in patients treated with doses ≥40 × 106 MSCs. In the QCT, the structural benefit was shown by a lower narrowing of the medial space,13 a reduction of the area of chondral defects,13 of the T2 map of the collagen composition17 and by an increase in cartilage thickness.11,13,18 These changes were reported one year after implant although the reduction of the PCI was observed from 6 months onwards.15 Like in the RCT, patients with the most consistent improvements were those who received the highest dose of MSCs (≥40 × 106).13,15,17,1
Safety resultsSafety data coincided in all studies. Intra-articular injection of MSCs was a well tolerated and safe intervention in all patients. Factors such as dose (high or low number of MSCs), administration regimen (one or more injections), source of MSCs (adipose tissue or bone marrow) and type of transplant (autologous or allergenic) did not alter favourable tolerance. Systemic reactions associated with treatment were not described, and only one case of hospital admission was reported, due to synovial fluid effusion which evolved favourably in 24 h.12 The most common side effect was the appearance of minor local reaction (pain and/or inflammation) in the puncture site (13%–53% of patients) (Table 2). In all cases, symptoms were temporary and treated locally with ice (occasionally with anti-inflammatories) in under 7 days. In the controlled studies, the incidence of these local effects did not present with any significant differences to those observed in patients treated with HA.12,14,19
DiscussionThe immunomodulator and regenerative potential of MSCs has positioned this type of therapy as one of the most promising in the therapeutic panorama of osteoarthritis. The use of stem cells in regenerative cartilage strategies is a highly appealing but also widely debated subject. This is the first SRL to analyse cellular treatment with quantifiable and phenotypically homogeneous MSCs implants. Our safety results coincide with previous studies4,5 and confirm that the expanded MSCs implant is a well tolerated and safe intervention, with no systemic toxicity. A significant percentage of patients suffered pain and/or local inflammation but this was temporary, insignificant and very similar to that of other intra-articular treatments. In general, it seems to occur more commonly in those patients treated with higher cellular doses and/or with higher injection volumes.
We were unable to reach a conclusion regarding the efficacy of MSCs from the evidence. The 9 concept test studies included, although prospective, were markedly biased by the low sample size, insufficient statistical power and lack of a control group. However, interesting conclusions were providing relating to their foundation and their possible utility in osteoarthritis. In this respect, the implant of MSCs showed a greater (and more sustained) clinical benefit than HA.14,19 This analgesic-functional improvement required a latency period greater than that observed with HA. Characteristically, analgesic improvement occurs at the beginning which precedes a more complete functional improvement. This early analgesia is a characteristic which has been confirmed by other studies,23 and has been linked to an initial paracrine and anti-inflammatory action of the implant. The potential of the MSCs self-renewal and differentiation would lead months later to the structural improvement observed at one year.24 From our results it may be deduced that the clinical and structural benefit produced by the MSCs may become quite sustained over time (up to 2 years)11,13,18 Recent studies appear to confirm this virtue, communicating stable clinical and structural improvements up to 4 years after the implant.25 One unanswered question from this review is the ideal cellular dose with which the maximum clinical and structural benefit may be obtained. Approximately, the most complete clinical improvements and the most notable radiologic changes were obtained with equal or higher doses to a 40 × 106 cells. This cut-off point appears to coincide with a range reported in the literature which, in general, inclines towards equal or higher doses.26 However, as occurs in some of the review trials,16 it is viable to say that there is analgesic improvement with a considerably lower doses.24 In view of this observation, it would appear that to obtain a paracrine and anti-inflammatory effect cellular implants do not need to be used in such large quantities.27 Regarding the radiologic benefit, our results confirm previous experiences which report an interesting regenerating effect of chondral damage.28 Singularly, this is governed by a response pattern which is in keeping with the improvement of functional parameters: significant from 6 months onwards and for a maximum of one year. The consistency of this pattern could prove that chondral damage repair is necessary to provide a more complete clinical and improvement which does not appear in the first few months, despite the analgesic benefit.
The results of this review should be interpreted with caution due to many limitations. To those already mentioned, such as the small sample size and main absence of a control group, we should add selection biases and poor classification, possibly accounted for by the fact that patients with the most severe osteoarthritis were those finally treated with MSCs. The absence of masking meant it was highly possible then there was the added existence of assessment biases, in which the intervention (treatment with MSCs) was particularly weighed by the assessor. Despite being a well-defined intervention, the great heterogeneity of dose and administration protocols was striking, making it difficult to compare different treatments. This heterogeneity is also applicable to the parameters which assessed radiologic damage. The huge variability of measures chosen and the low sample seize impeded the drawing of consistent conclusions regarding the magnitude of the benefit or the determination of a dose-response pattern.
To sum up, this review proposes a basis for the therapeutic use of expanded MSCs in osteoarthritis as a modifying treatment of symptoms and structure. Its efficacy in clinical trials has been confirmed and it is a safe technique for patients, and is viable for rheumatologists. However, to become a real and accessible alternative, MSCs implants must also overcome other logistic and financial barriers. In this sense the good safety results of the allergenic implants in the review, validate previous experiences.29 They provide interesting clinical information in favour of the bio bank implants, at a lower cost, without discomfort for the patient and with higher logistic advantages. Among these is access to MSCs from the umbilical cord which are widely available and also (potentially) effective in osteoarthritis of the knee.30,31 We will have to wait for the results of ongoing clinical trials to confirm whether this preliminary promising evidence materialises as consistent and reproducible benefits.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Álvarez Hernández P, de la Mata Llord J. Tratamiento de la artrosis de rodilla con células mesenquimales estromales expandidas: revisión sistemática de la literatura. Reumatol Clin. 2022;18:49–55.