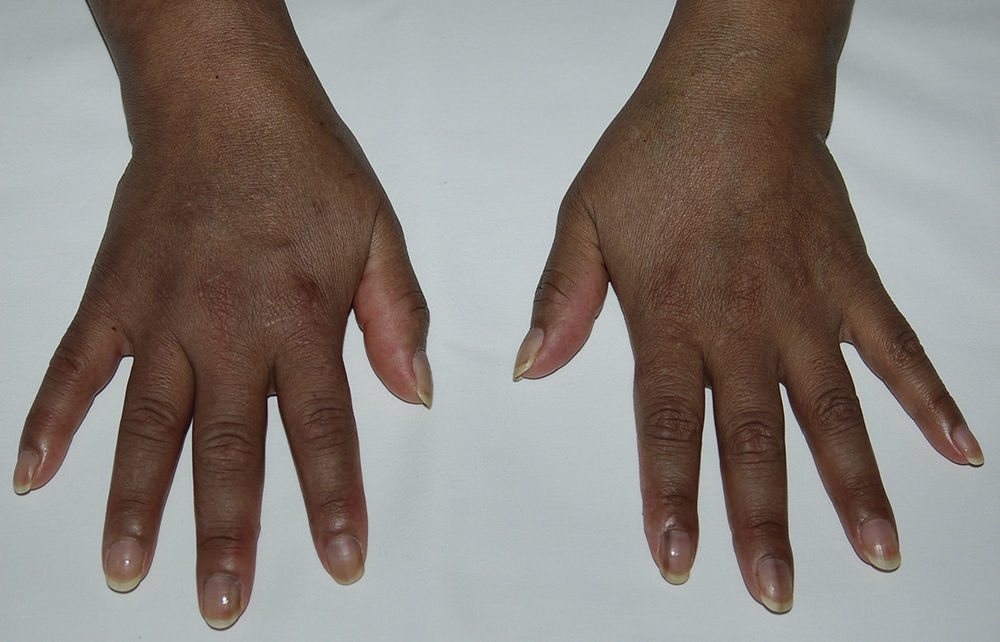
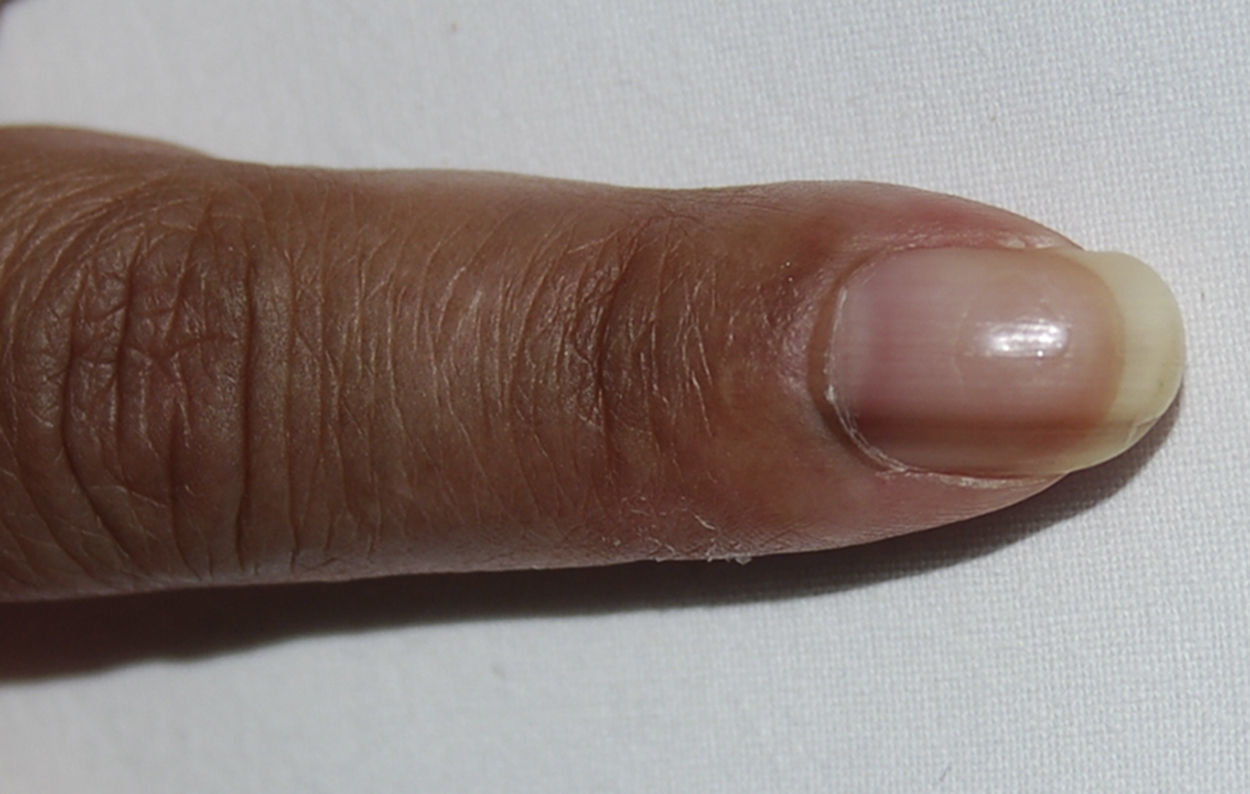
We present the case of a 48-year-old-woman from Ecuador who was diagnosed with systemic lupus erythematosus in March 2004 based on symmetric polyarthritis which affected the wrists and small joints of the hands, as well as oral ulcers, Coombs-positive hemolytic anemia, leukopenia and positive antinuclear, anti-DNA, anti-Sm, antiribonucleoprotein, anti-Ro/SSA and IgM and IgG anticardiolipin antibodies. In May 2004 she developed proteinuria and a renal biopsy was compatible with class IIIC lupus nephritis (focal sclerosing).1 Prednisone was started at 1mg/kg/day in a descending pattern as well as azathioprine 100mg/day, with good clinical response, but the latter was temporarily suspended in May 2009 due to herpes zoster and definitively in October 2010 due to persistent lymphopenia. At that time hydroxychloroquine was added at a dose of 200mg/day, but the patient developed hyperpigmentation after 12 months on the drug. Physical examination showed a generalized bluish-gray pigmentation, which was more intense on the face and back of the hands (Fig. 1), as well as longitudinal bands of similar characteristics on the nails of the second finger of the left hand and the third finger on both hands (Fig. 2). No evidence of hyperpigmentation of the mucosal surfaces was seen. Laboratory analyses, including blood chemistry, hemogram, hormones (thyroid stimulating hormone, cortisol, ACTH) and acute phase reactants were within normal ranges. After reasonably ruling out other causes of generalized hyperpigmentation, including Addison's2 disease, this event was attributed to treatment with hydroxychloroquine. However, we decided to continue the drug, in spite of the esthetic considerations, due to the high risk of SLE reactivation after its withdrawal,3 with the pigmentation remaining stable during follow up and without the development of ocular manifestations.
Antimalarials, chloroquine and hydroxychloroquine, are first line drugs for the treatment of SLE due to their capacity to prevent relapses, including severe ones, in addition to their adjuvant effect in inducing or maintaining remission, improving the metabolic profile, reducing the thrombotic risk and not inducing immunosuppression.4 Adverse events related to these drugs are generally mild and reversible, with mucocutaneous hyperpigmentation being one of the most common ones, occurring in up to 10–25% of cases.5 This event is dose-dependent and may affect the chin, face, hard palate, trunk and nailbeds.6 At the skin level it presents as maculae or plaques that vary in color from gray-blue to dark purple and which appear after approximately 4 months of treatment.7 In our case, hyperpigmentation was diffuse and did not follow a common distribution. However, cases have been described with extensive affected areas that cover the trunk and extremities completely.8 Nail affection is less common and may manifest as hyperpigmented bands (longitudinal melanonychia) or be diffuse.9 Skin pigmentation tends to remit slowly in the months after drug suspension,5 but nail lesions may last for years, although in a less intense manner.10 The pigmentation mechanism associated with these drugs is unknown but its affinity for melanine may account for its depositing in skin.6 When performing the differential diagnosis of diffuse cutaneous hyperpigmentation one must take into account metabolic problems (hemochromatosis, porphyria cutanea tarda, pellagra), neoplasia (lung carcinoma, metastatic melanoma, mycosis fungoides), endocrine disorders (Addison's disease, Nelson's disease, ectopic ACTH secretion syndrome, hyperthyroidism), autoimmune diseases (systemic sclerosis, primary biliary cirrhosis), heavy metal intoxication (gold, silver) and drugs (colpromacin, amiodarone, tetracycline).2
Ethical ResponsibilitiesProtection of Persons and AnimalsThe authors state that no experiments were performed on humans or animals for this study.
Data ConfidentialityThe authors state that all of their workplace protocols on the publication of patient data have been followed and that the patients included have received enough information and have given written informed consent to participate in the study.
Right to Privacy and Informed ConsentThe authors state that they have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in possession of the corresponding author.
Conflict of InterestThe authors declare no conflict of interests.
Please cite this article as: Sifuentes Giraldo WA, Grandal Platero M, de la Puente Bujidos C, Gámir Gámir ML. Hiperpigmentación cutánea generalizada y melanoniquia longitudinal secundarias al tratamiento con hidroxicloroquina en lupus eritematoso sistémico. Reumatol Clin. 2013;9:381–2.