To assess the prevalence of gallstone disease and identify associated risk factors in rheumatoid arthritis (RA) patients compared to the general population.
MethodsEighty-four women with rheumatoid arthritis were included in the study. Each patient was assessed via a structured interview, physical examination, abdominal ultrasound and blood test including lipid profile. The prevalence of gallstone disease in rheumatoid arthritis was compared with data from a study of the Spanish population matched by age groups.
ResultsTwenty-eight of the 84 women had gallstone disease (33.3%). RA women with and without gallstone disease were similar in most of the variables assessed, except for older age and menopausal status in the former. A greater prevalence of gallstone disease was seen in rheumatoid arthritis patients compared to the general population of the same age; however, the differences were significant only in women aged 60 or older (45.5% versus 23.1% respectively, P-value .008). The age-adjusted OR of developing gallstone disease in RA women compared with general population women was 2,3 (95% CI: 1.3–4.1). A significantly higher HDL3-c subfraction and higher apoA-I/HDL and HDL3-c/TC ratios were observed in patients with gallstone disease.
ConclusionWomen with rheumatoid arthritis may have a predisposition to gallstones that can manifest in middle or older age compared with women in the general population. This situation could be related to chronic inflammation and HDL metabolism.
Evaluar la prevalencia de litiasis biliar e identificar los factores de riesgo asociados en pacientes con artritis reumatoide (AR) en comparación con la población general.
MétodosOchenta y cuatro mujeres con AR fueron incluidas en el estudio. Cada paciente fue evaluada a través de una entrevista estructurada, un examen físico, una ecografía abdominal y un análisis de sangre que incluía el perfil lipídico. La prevalencia de litiasis biliar en AR se comparó con los datos de un estudio de la población española emparejada por grupos de edad.
ResultadosVeintiocho de las 84 mujeres tenían litiasis biliar (33,3%). Las pacientes con y sin colelitiasis fueron similares en la mayoría de las variables evaluadas, a excepción de la edad más avanzada y mayor prevalencia de estado menopáusico en las pacientes con AR. Las pacientes con AR presentaban una mayor prevalencia de litiasis biliar en comparación con la población general de la misma edad; sin embargo, estas diferencias solo fueron significativas en mujeres de 60 años o más (45,5% vs. 23,1% respectivamente, p-valor 0,008). La OR ajustada por edad de presentar litiasis biliar en mujeres con AR respecto a mujeres de la población general fue de 2,3 (IC del 95%: 1,3-4,1). Se observó una subfracción de c-HDL3 significativamente más alta y una relación mayor de apoA-I/HDL y c-HDL3/TC en las pacientes con litiasis biliar.
ConclusiónLas mujeres con AR pueden tener una mayor predisposición a la presencia de litiasis biliar en comparación con las mujeres en la población general, sobre todo en edades más avanzadas. Esta situación podría estar relacionada con la inflamación crónica y el metabolismo de las HDL.
In recent decades, the presence of comorbidities in patients with rheumatoid arthritis has become more pronounced since the new therapies have changed the natural course of the disease.1 Although one of the most significant comorbidities is cardiovascular disease, as a result of a process of accelerated atheromatosis in the context of inflammation and lipid metabolism disorders, other disorders are also common.2 Alterations in lipid metabolism, both quantitative and qualitative, observed in these patients have been linked to the inflammatory process itself, although the mechanisms involved have not been well defined.3 Nevertheless, altered lipid metabolism as well as inflammation can affect other organs or systems.4 In this regard, it is known that, in the context of inflammation, the endothelial function of the gallbladder is altered, and consequently the recycling of lipids could be decreased.5 At the same time, it is known that one of the main risk factors involved in the pathogenesis of cholesterol gallstones, which constitute 80% of stones,6 is alterations in lipid metabolism.7,8 Thus, patients with chronic inflammatory disease may present a higher prevalence of gallstones. In fact, in a small number of studies, a higher prevalence of gallstones has been observed in patients with systemic lupus, antiphospholipid syndrome, and rheumatoid arthritis.9,10 In addition, other conditions associated with an increased incidence of gallstones such as age, female gender, sedentary lifestyle and, possibly, chronic intake of non-steroidal antiinflammatory drugs (NSAIDs)11 are present in rheumatoid arthritis. The purpose of this study was to assess the prevalence of gallstones in patients with established rheumatoid arthritis compared with the general population, and its relationship with lipid metabolism disorders and other risk factors.
MethodsPatientsOne hundred and eight patients with rheumatoid arthritis were consecutively enrolled in this cross-sectional study from the outpatient rheumatology clinic at the Hospital Universitari de Bellvitge, Barcelona. The manuscript has been accepted for its publication by the Clinical Research Ethics Committee of Bellvitge University Hospital. The data of the patients were anonymized for the purposes of this analysis. The confidential information of the patients was protected according to national normative. All patients met the 1987 revised criteria for RA of the American College of Rheumatology.12
Each patient who agreed to participate was systematically assessed through a self-reported questionnaire, a structured interview and a general physical examination. Blood samples were obtained for routine biochemistry and hematologic tests. An abdominal ultrasound was performed in the days following the clinical and biochemical study. The lipid profile included: total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), very low-density lipoprotein cholesterol (VLDL-c), high-density lipoprotein cholesterol (HDL-c), subfraction 2 of high-density lipoprotein cholesterol (HDL2-c), subfraction 3 of high-density lipoprotein cholesterol (HDL3-c), apolipoprotein A-I (apoA-I), apolipoprotein B (apoB) and lipoprotein (a) [lp(a)]. Clinical variables reflecting RA activity including patient overall assessment, physical disability score using the Health Assessment Questionnaire (HAQ),13 acute-phase reactant levels, number of tender and swollen joints and duration of morning stiffness were assessed. Disease activity was measured using the Disease Activity Score based on the evaluation of 28 joints (DAS28).14 Additionally, data on RA disease duration, menopausal status and history of gastrectomy were collected. The medical records of all patients were reviewed. Height and weight were measured and body mass index was calculated. Sedentary lifestyle was considered if patients did not walk 30min without stopping at least three times a week.
Determination of Serum Lipid ConcentrationsBlood specimens were obtained after a fast of at least 12h, collected into tubes without anticoagulant, centrifuged at 1200g for 10min at room temperature and stored at 4°C until analysis. A modular system analyzer (Roche Diagnostics, Basel, Switzerland) was used to perform all procedures, which are specified below. Serum TC and VLDL were isolated by preparative ultracentrifugation at a density gradient of 1006kgL−1 and their cholesterol content was quantified by an enzymatic colorimetric method (CHOD-PAP, Roche Diagnostics, Basel, Switzerland), such as used for measurement of TG concentrations (GPO-PAP, Roche Diagnostics, Basel, Switzerland). A direct colorimetric enzymatic method was performed to quantify the serum HDL-c concentrations (HDL-c plus, Roche Diagnostics, Basel, Switzerland). Serum HDL3-c concentrations were measured in the supernatant obtained after precipitation of the HDL2 subfraction through the addition of a 15% polyethylene glycol solution (PEG 20 000) at pH 7.5. The difference between concentrations of HDL-c and HDL3-c was applied to calculate the serum HDL2-c concentrations, and the Friedewald equation was used to estimate the serum LDL-c concentrations (although it was considered inaccurate when TG level exceeded 2.3mmolL−1). Finally, ApoA-I, apoB and lp(a) were quantified by immunoturbidimetric methods (Roche Diagnostics, Basel, Switzerland).
Abdominal UltrasoundUltrasonography was performed by a trained operator unaware of the medical history of the patients, except that all had RA. The examination was performed with the subject in the supine and left lateral decubitus positions, after a fast of at least 6h. The gallbladder was examined in both longitudinal and transverse planes using a real-time, linear array scanner with a 3.5MHz probe. Ultrasonographic findings were classified as follows: normal gallbladder (lumen without echoes), presence of gallstones and absent gallbladder (non-visualization of the gallbladder lumen without high-density echoes and acoustic shadow in the gallbladder fossa).
Gallstone disease was defined as the presence of gallstones on ultrasonography or cholecystectomy. Cholecystectomy was defined as a positive patient history of cholecystectomy in which, furthermore, the gallbladder lumen was not visualized on ultrasonography.
Statistical AnalysisThe Statistical Package for Windows 18.0 (SPSS, Chicago, IL) was used to analyze the data. Normality of variables was tested by the Kolmogorov–Smirnov test. Normally-distributed continuous variables were analyzed using an analysis of variance (ANOVA) or analysis of covariance (ANCOVA). Continuous variables not normally distributed and ordinal variables were compared with non-parametric tests (Mann–Whitney U test). Categorical variables were analyzed by chi-square test or Fisher's exact test. P-values less than .05 were considered significant. Results of categorical and ordinal variables are shown as relative frequency (%), continuous variables are expressed as mean±standard deviation (SD) and continuous variables not normally distributed by median (interquartile range).
Data from a study of the Spanish population15 consisting of 275 women aged between 30 and 79 years were used to compare the prevalence of GS in patients with RA matched by age groups.
The association between RA and gallstone disease (which includes gallstones and cholecystectomy) has been analyzed using odds ratio (OR) calculated by logistic regression analysis. A model has been made for each age group, another for an overall group and another adjusted for age (<60 years versus≥60 years).
ResultsOf 108 patients with RA, 84 were women and 24 men. Only two men had gallstone disease and none had undergone cholecystectomy. Therefore, only women were included in the study. The main demographic, clinical and laboratory features of RA participants are summarized in Table 1. Patients in the two groups, with and without gallstone disease, were similar in most variables assessed, except for age and menopausal status, which were significantly greater in the patients with GD. None had a history of gastrectomy.
Clinical Characteristics of Female RA Patients.
| Gallstone disease (n=28) | No gallstone disease (n=56) | P-value | |
|---|---|---|---|
| Age, yrs | 65 (9) | 58 (12) | .007 |
| Disease duration, yrs | 11 (8) | 13 (9) | .343 |
| Rheumatoid factor+% | 71 | 84 | .179 |
| Nodular disease, % | 21 | 36 | .182 |
| Erosive disease, % | 81 | 81 | .969 |
| HAQ 0–3 | 1.41 (0.9) | 1.38 (0.8) | .900 |
| DAS 28 | 4.5 (1.6) | 5.0 (1.3) | .204 |
| Current smoker, % | 4 | 5 | 1.000 |
| Systolic blood pressure, mm Hg | 158 (23) | 148 (25) | .075 |
| Diastolic blood pressure, mm Hg | 88 (9) | 85 (10) | .245 |
| Body mass index, kg/m2 | 28.3 (4) | 27.5 (4) | .491 |
| Sedentary, % | 29 | 29 | 1.000 |
| Menopausal, % | 93 | 73 | .035 |
| ESR, mm/h | 38 (19) | 37 (23) | .924 |
| CRP, mg/la | 11 (3–22) | 8 (4–27) | .757 |
| Glucose, mmol/l | 5.3 (0.8) | 5.2 (0.9) | .669 |
| GGT, μkat/La | 0.28 (0.22–0.59) | 0.26 (0.19–0.41) | .220 |
| ALP, μkat/La | 1.4 (1.1–1.8) | 1.2 (1.0–1.6) | .115 |
| Creatinine, μmol/L | 78 (14) | 83 (22) | .281 |
| RA treatment, %: | |||
| Glucocorticosteroids | 82 | 84 | .836 |
| Methotrexate | 64 | 47 | .142 |
| TNF-alpha blockers | 14 | 9 | .478 |
| Other disease-modifying antirheumatic drugs | 21 | 44 | .046 |
HAQ: Health Assessment Questionnaire; DAS: Disease Activity Score; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; GGT: gamma-glutamyl transferase; ALP: alkaline phosphatase; TNF: tumor necrosis factor.
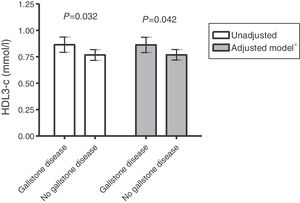
Serum lipid, lipoprotein and apolipoprotein values in women with RA and in controls are shown in Table 2. No significant differences in TC, LDL-c and its main protein, apoB, values were found between groups. However, although no significant differences were found in plasma levels of HDL-c and its main protein, apoA-I, between patients with or without gallstone disease, a significantly higher HDL3-c subfraction and higher apoA-I/HDL and HDL3-c/TC ratios were observed in the former. This difference in the HDL3-c subfraction remained after adjustment for age and triglycerides (Fig. 1).
Comparison of Serum Lipid, Lipoprotein and Apolipoprotein Values in Female RA Patients With and Without Gallstone Disease.
| Gallstone disease (n=28) | No gallstone disease (n=56) | P-value | |
|---|---|---|---|
| Cholesterol, mmol/l | 5.47 (0.85) | 5.60 (0.96) | .528 |
| Triglyceride, mmol/la | 1.20 (0.90–1.70) | 1.05 (0.80–1.40) | .405 |
| LDL-c (mmol/l) | 3.21 (0.76) | 3.41 (0.82) | .286 |
| VLDL-c, mmol/la | 0.26 (0.20–0.40) | 0.22 (0.13–0.37) | .180 |
| HDL-c, mmol/l | 1.93 (0.36) | 1.89 (0.53) | .685 |
| HDL2-c, mmol/l | 1.08 (0.30) | 1.08 (0.49) | .943 |
| HDL3-c, mmol/l | 0.86 (0.13) | 0.77 (0.20) | .032 |
| Apolipoprotein A-I, g/L | 1.76 (0.25) | 1.65 (0.34) | .129 |
| Apolipoprotein B, g/L | 0.89 (0.21) | 0.92 (0.19) | .488 |
| Lipoprotein (a), g/La | 0.34 (0.15–0.68) | 0.34 (0.14–0.65) | .655 |
| HDL-c/cholesterol | 0.36 (0.08) | 0.34 (0.09) | .292 |
| ApoA-I/HDL | 0.33 (0.06) | 0.30 (0.06) | .038 |
| HDL3-c/cholesterol | 0.16 (0.03) | 0.14 (0.04) | .036 |
LDL-c: low-density lipoprotein cholesterol; VLDL-c: very-low-density lipoprotein cholesterol; HDL-c: high-density lipoprotein cholesterol; ApoA-I: apolipoprotein A-I.
The prevalence of gallstone disease stratified by age in RA patients and controls is shown in Table 3. Female RA patients aged 60 or over had a significantly higher prevalence of gallstone disease compared to the general population of the same age. It could also been observed, that these patients present a higher prevalence of cholecystectomy than controls.
Comparison of Gallstone Prevalence by Age Groups Between RA and Controls.
| Age (years) | Female participants | Gallstones | Cholecystectomy | Gallstone disease | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RA | Control | RA | Control | P-value | RA | Control | P-value | RA | Control | P-value | |
| 30–39 | 4 | 63 | 0% (0) | 7.9% (5) | 1.000 | 0% (0) | 3.2% (2) | 1.000 | 0% (0) | 11.1% (7) | 1.000 |
| 40–49 | 12 | 61 | 25% (3) | 9.8% (6) | .161 | 0% (0) | 3.3% (2) | 1.000 | 25% (3) | 13.1% (8) | .374 |
| 50–59 | 24 | 60 | 8.3% (2) | 10% (6) | 1.000 | 12.5% (3) | 1.7% (1) | .068 | 20.8% (5) | 11.7% (7) | .210 |
| ≥60 | 44 | 91 | 11.4% (5) | 13.2% (12) | .765 | 34.1% (15) | 9.9% (9) | .001 | 45.5% (20) | 23.1% (21) | .008 |
| Total | 84 | 275 | 11.9% (10) | 10.5% (29) | .726 | 21.4% (18) | 5.1% (14) | <.001 | 33.3% (28) | 15.6% (43) | <.001 |
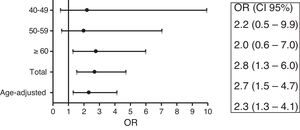
Female RA patients ages 60 or over, compared to controls, presented an OR of 2.8 (95% CI: 1.3–6.0) (Fig. 2) to have gallstone disease. Adjusting for age female RA patients had an OR of 2.3 (95% CI: 1.3–4.1).
DiscussionTo our knowledge, this is the first study to assess the prevalence of GD in patients with RA in western countries. It shows that female patients with RA ≥60 years of age had a significantly higher prevalence of gallstone disease compared to the general population of the same age and gender. This high prevalence is independent of age (OR age-adjusted 2.3). General population studies have shown age and female sex to be among some of the most important risk factors for developing GD.16 Thus, as would be expected, older and menopausal RA women would have a higher prevalence of GD. Nevertheless, the present study shows that this prevalence is higher than expected in the general population compared for age and gender.
Biliary lithiasis in RA has received little attention and few studies have been conducted on this topic. A study by Ito et al. in 224 RA patients in Japan (182 female)9 showed a significantly higher incidence of gallstones (including post-cholecystectomy patients) mainly cholesterol stones in female RA patients than in female control (15.4% and 5.2%, respectively). In that study, RA patients were older and had an increased incidence of hypercholesterolemia than controls and, in agreement with our study, the prevalence of GD in male RA patients was very low. Pamuk et al. studied the prevalence of GD in 113 RA patients in Turkey (92 female)10 and possible related factors. That study showed female RA patients to have a significantly higher prevalence of gallstone disease (including cholecystectomy) compared to controls (22.8% and 11.7%, respectively) and only older age was significantly associated with the presence of GD in RA. In our study, female RA patients also had a higher prevalence of GS than controls, although higher than that observed in the abovementioned studies (33.3% in RA and 15.6% in controls). This difference could be explained by the fact that GD is more common in western countries and its incidence continues to rise.11
Gallstone disease is a common digestive disorder worldwide,17 and, although there are different types of gallstones depending on their composition, most biliary stones in western societies are made of cholesterol, which has been reported in up to 80%–90% of gallstones found at cholecystectomy.18 Even though GD is a multifactorial disease derived from complex interactions between many genetic and environmental factors, impaired cholesterol metabolism has been one of the mechanisms involved in the formation of cholesterol gallstones.19 The first and essential requirement is the supersaturation of bile in cholesterol; however, enhanced cholesterol nucleation, impaired gallbladder emptying with stasis and intestinal hypomotility have also been associated.20 In supersaturated bile, phospholipids solubilize cholesterol into vesicles, which may precipitate in monohydrate crystals and become entrapped in gallbladder mucin gel with bilirubinate, and finally agglomerate into a macroscopic gallstone. Hence, cholesterol gallstone, composed predominantly of cholesterol crystals, results from a biochemical imbalance between lipids and bile salts in the gallbladder bile. Notwithstanding, no definitive association trends between serum lipids and gallstones have been found, except for a high frequency of gallstones in subjects with hypertriglyceridemia (type IV hyperlipoproteinemia).21,22 It has been described that nearly all patients with hypertriglyceridemia have supersaturated gallbladder bile even if they are slim. In this respect, female RA patients with GS in the present study displayed a higher plasma TG level compared to those without GD; however, the differences were not statistically significant. On the other hand, it has been suggested that the gallstone risk varies inversely with plasma total HDL or HDL3 cholesterol, attributing to HDL cholesterol a protective effect against gallstone formation.23,24 Furthermore, some studies in mice showed a genetic link between bile acid synthesis and HDL-cholesterol levels through the gene that regulates the expression of the cholesterol 7-alpha hydroxylase (the rate-limiting enzyme for bile acid biosynthesis and cholesterol elimination).20 Other studies found no association between serum HDL-c concentrations and gallstone disease.15 In line with this, our patients with gallstones had a slightly higher plasma concentration of total HDL-c than those without gallstone disease, although these differences were not statistically significant. By contrast, female RA patients with gallstone in the present study displayed significantly higher HDL3-c levels, even after adjustment for age and triglycerides and apoA-I/HDL and HDL3-c/TC ratios than those without gallstone disease. HDL-c is a source of biliary cholesterol, which forms gallstones.25 Nascent HDL particles are transformed into HDL3 and HDL2 particles as they acquire cholesterol esters from other lipoproteins and cells. In turn, the large and spherical HDL2 particles are transformed into the smaller HDL3 particles after delivering their cholesterol content to the liver where this cholesterol is incorporated into bile. Thus, a higher HDL3-c concentration in patients with GS could be related to a higher rate of liver cholesterol delivery from HDL.
Other studies support the hypothesis of increased biliary catabolism of cholesterol in subjects with gallbladder disease characterized by lower ApoB and higher ApoA-I serum concentrations.26 RA patients with gallstone disease in this study also showed lower ApoB and higher ApoA-I values, but no significant differences were found compared with those without gallstone disease. However, RA patients with GS display a higher apoA-I/HDL-c ratio than those without gallstone disease, which may be explained by the higher protein/lipid ratio of HDL3 particles.
In addition to an excess supply of cholesterol in the liver, impaired gallbladder emptying with stasis could contribute to the formation of gallstones.20 Biliary lipid secretion is regulated by an elaborate network of ATP-binding cassette (ABC) transporters on the hepatocyte canalicular membrane.19 It has been observed that the expression and function of ABC transporters could be decreased in liver in adjuvant-induced arthritis rats27 as an animal model for inflammation, thereby hindering the secretion of bile salts and favoring the formation of gallstones.
Furthermore, higher blood concentrations of inflammatory cytokines (IL-1α, IL-6 and TNF-α) in menopausal women with GS have been observed.28 Moreover, it has been reported that the endothelial functions of the gallbladder for recycling of lipids could be diminished in an inflammatory context.5 Thus, the chronic inflammatory state present in RA patients, related to lipid profile and gallbladder function, might explain the higher prevalence of GS in older female RA patients.
Albeit not the purpose of this study, a further major aspect to mention is that an association of gallstones with cardiovascular disease has been suggested.29 This would be very interesting to consider, taking into account that patients with RA have increased cardiovascular risk compared to the general population,30 as has been widely reported in the literature.
A potential limitation of this manuscript is that the data obtained was compared with the information available from a study of several years. Nevertheless, to our knowledge, it is the only existing reference up to the date in the Spanish population.
ConclusionsWomen with RA may have a predisposition to gallstone disease that can manifest in middle age or older compared with women in the general population. This situation could be related to chronic inflammation and HDL metabolism. Gallstone disease could be considered a new co-morbidity in female RA patients which has received little attention, although it contributes greatly to health care costs and patient morbidity.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestNone of the authors has any conflicts of interest.
The authors thank Dr. J. Valverde, Emeritus Professor of Rheumatology, for this contributions and critical review of the manuscript, and Christine O’Hara for help with the English version of the manuscript.