Methotrexate (MTX) is the first choice disease modifying anti-rheumatic drugs for rheumatoid arthritis. In spite of its generalized use by rheumatologists worldwide, there is a general lack of agreement regarding the route of administration, the start-up dose and the way to increase the same. In this article, we propose a simplified outline for the use of the drug that should be individualized, based on it's pharmacological aspects, guidelines and recommendations published in high impact factor journals during the past few years. Adverse reactions and side effects, as well as their follow up are also reviewed.
El metotrexato (MTX) es el fármaco modificador de la enfermedad de primera elección en artritis reumatoide. A pesar del uso casi generalizado por reumatólogos en todo el mundo, hay mucha discordancia entre la forma de iniciar la dosis, la vía de administración y la forma de realizar el incremento de dosis. En este artículo se planteamos un esquema simplificado del uso de este fármaco a individualizar en cada caso, basado en los aspectos farmacológicos, guías y protocolos de manejo publicados en revistas de impacto de nuestra especialidad en los últimos años. Se revisa además las reacciones adversas y efectos secundarios y cómo realizar el seguimiento de éstos.
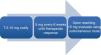
Rheumatoid arthritis (RA) is an autoimmune systemic disease characterized primarily by symmetric, episodic, chronic, erosive, deforming polyarthritis that produces long-term joint disability if uncontrolled. Currently, methotrexate (MTX) is the disease-modifying drug most commonly used in RA and the first treatment choice.1–3 Despite its almost universal therapeutic application, there is much disagreement on the part of rheumatologists in terms of the starting dose, how to increase it and the route of administration (Fig. 1).
In this article we propose a simplified clinical setting which may serve to identify general recommendation for each individual case. Our proposal is based on the analysis of review articles, meta-analysis, consensus and clinical practice guidelines published in the last five years in influential journals of our specialty.
How Does It Work?MTX is an antimetabolite that competitively inhibits dihydrofolate reductase. This enzyme participates in the formation of tetrahydrofolate, necessary for the formation of the nucleoside thymidine, required for the synthesis of DNA, RNA, thymidylate and proteins. It partially inhibits the immune response4 and, although through a poorly understood mechanism, autoimmune joint inflammation is reduced in the long term.
What Pharmacological Aspects Must Be Taken into Account?Oral absorption of MTX is dose dependent and varies significantly according to intestinal transit. Meals, diarrhea and non-absorbable antibiotics decrease its absorption, while constipation increases it. The oral mean bioavailability is 33% and parenteral is 77%. Once in the serum, 50% circulates bound to proteins, with a half life of 3–10h. Excretion is 90% renal and 10% gastrointestinal.5 These are issues that we must take into account when assessing the risk of adverse reactions and side effects of the drug, as their frequency increases in proportion to plasma levels (Table 1).
Main Interactions of Methotrexate.
| Amoxicillin | Indomethacin |
| Trimethoprim | Nabumethone |
| Triamterene | Doxycycline |
| Omeprazole | Penicillin G |
| Tamoxifen | Flurbiprofen |
| Ketoprofen | Aspirin |
| Clotrimoxazole | Tazobactam |
| Piroxicam | Sulphamethoxazole |
| Diflunisal | Mercaptopurine |
| Ketorolac | Etodolac |
| Diclofenac | Acyclovir |
| Piperacillyn | Phenylbutazone |
| Naproxen | Citarabin |
| Kanamycin |
Modified from http://www.drugs.com/methotrexate.html.
One should begin treatment with disease modifying drugs as soon as the diagnosis is carried out.2 Diagnosis is mostly based on the history and physical examination, and less on the classification criteria. The patient will usually complain of pain, morning stiffness and swelling. Physical examination reveals symmetrical swelling and joint tenderness. However, these findings are not unique to RA in its early stage and a differential diagnosis is necessary. The RA classification criteria published in September 2010 by the ACR/EULAR6 aim to classify patients who may benefit from treatment in the initial stages of the disease.
What Tests Should Be Performed Before Onset of Treatment?Patients should be screened for assessment of potential toxicity risk factors (such as alcohol) and CBC, CRP and ESR, serum creatinine, transaminases, albumin, antibodies against hepatitis B and C, rheumatoid factor, anti cyclic citrullinated peptide antibodies, lipid profile, pregnancy test and chest, hands and feet X-rays carried out.1,3 The application of the vaccine against pneumococcus and seasonal influenza virus1 is also recommended. HIV serology3 should also be assessed (Table 2).
The patient and the physician should assess pain and/or global disease activity scores (visual analog scale of 0–10). The doctor should perform a 28 joint count calculating a standard index of disease activity such as the DAS28. If positive for activity (DAS28 greater than 3), treatment is indicated.
What Dose Should Be Used at Onset of Treatment?There are multiple ways to approach treatment and each rheumatologist has his or her own way home, especially with regard to the route of administration and increasing the therapeutic dose. Here, we refer to treatment consensus and systematic reviews.1–3,7 Traditionally, the recommended starting dose has been of 7.5–10mg weekly for 4 weeks associated with folic acid in doses of 5–10mg the day after taking MTX. Subsequently, a progressive increase between 2.5 and 5mg every 2–4weeks until a dose of 25mg is reached between 3 and 6 months from the onset of treatment,5 since high doses of 25–30mg weekly are more effective as disease-modifying than 10–15mg3.
The starting dose recommended by GUIPCAR-SER typically varies between 7.5 and 15mg weekly orally (4–6 tablets of 2.5mg).1 After 15mg it is recommended to use the intravenous route to improve bioavailability (33% oral vs 77% parenteral).1
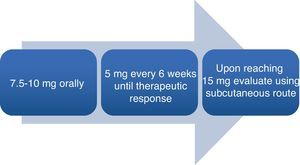
However, a systematic review of the literature on the use of MTX3 recommended starting treatment with 10–15mg orally, increasing 5mg every 2 weeks up to 20–30mg, depending on clinical response and tolerance. Parenteral administration of the drug should be considered in the case of poor response or intolerance.
Furthermore, the effectiveness of drug response, including a joint count, should be monitored,8 for example using the DAS28, which has been validated and reviewed for this purpose in recent years.9
What Adverse Reactions and Side Effects Can We Foresee?The evidence of risk factors for severe MTX toxicity suggests that a creatinine clearance of less than 79ml/min increases the risk of pulmonary toxicity, and severe hypoalbuminemia in these patients is associated with liver and lung toxicity.10,11 Abnormalities on chest x-rays, rather than respiratory function tests, are predictive of an increased risk of MTX pneumonitis.12,13 The subgroups of patients with additional risk of developing liver failure secondary to the drug are mainly obese, diabetics and patients with viral or alcoholic hepatitis.14,15 Adding this observational evidence to that of experts on the contraindications of the drug in randomized clinical trials in the last 15 years, it is not recommended to use MTX in the presence of significant renal disease, liver disease, leukopenia of less than 3000/mm3, thrombocytopenia of less than 100,000/mm3, age over 70 years, malignancy, pregnancy or fertility problems, a history of substance abuse/alcoholism, chronic lung disease and acute or chronic systemic infections (Table 3).
The toxicity of oral MTX is dose dependent, and initial doses of 20mg have shown greater efficiency but a higher incidence of intolerance. Initial doses of 12.5–20mg weekly compared to oral doses of 5–10mg have shown to be more effective without increasing toxicity.16 Regarding administration, the parenteral route has proven more effective in retrospective studies and shows less gastrointestinal adverse reactions most probably due to greater bioavailability.17,18
How and Why Folic Acid Is Used in These Patients?We recommend the prescription of at least 5mg of folic acid week. Folic acid supplementation significantly reduced liver and gastrointestinal toxicity without affecting efficacy.19 Similarly, doses higher than 5mg weekly of folinic acid were associated with a greater number of swollen joints and, therefore, a decreased efficacy of the drug for disease control.20,21
What Controls Should Be Used for Safety Monitoring?When you start MTX and increase its dose, ALT and AST, creatinine and CBC controls should be performed every month or 6 weeks until the maintenance dose is reached and then every 1–3 months, given that high levels of AST have been linked to higher incidence of hepatotoxicity in RA. Risk factors for toxicity and adverse reactions should be assessed in each visit.3 Creatinine clearance should be monitored, since renal function abnormalities are associated with increased lung toxicity. The CBC should be used to monitor the possibility of blood disorders (Table 4).
In What Cases Should MTX Be Discontinued Due to Toxicity/Adverse Reactions?MTX should be discontinued if AST exceeds more than three times the upper limit of normal. However, it may be reintroduced at a lower dose once normal. If the AST/ALT is more than three times the normal limit, the dose should be adjusted. If after discontinuation of MTX, AST/ALT remains more than three times the upper limit of normal, diagnostic testing should be conducted3 to justify it.
The evidence suggests that elevations of ALT are common but transient, and also finds that significant multiple ALT elevations are more indicative of a liver biopsy that a single reading. Cirrhosis/liver fibrosis secondary to MTX are rare. Experts recommend ruling out other causes of elevated ALT, such as NSAIDs, obesity and chronic alcohol intake before performing a liver biopsy, if enzyme elevation persists after cessation of the drug.3
Is Long-term Treatment of Rheumatoid Arthritis With Methotrexate Safe?Based on its acceptable safety profile, MTX is suitable for long-term use. Patients with RA have increased mortality compared to the population in general.22 However, patients with RA treated with MTX had a lower incidence of mortality than patients with RA without MTX and less cardiovascular mortality in a large prospective study with a follow up of 6 years.23 In addition, in two case-control studies, MTX was shown not to be a risk factor and even was a protective factor for cardiovascular disease. In the long term, MTX was not associated with a higher rate of serious infections, including herpes zoster.
Although patients with RA have an increased risk of lymphoma compared with the general population, the risk of MTX independent from the evidence of RA is inconclusive because the studies did not address RA as the reference population and the risk was not adjusted for the severity of the disease. Five case-reports suggest that MTX may be associated with lymphoproliferative disease related to Epstein-Barr virus and reported the regression of disease after the withdrawal of the drug.24
In Patients Without Prior Treatment With Disease Modifying Drugs, Can It be Used as Monotherapy or Should It Be Combined?The effectiveness/toxicity balance of MTX alone favors the combination of MTX with another disease modifying drug. MTX should be considered as the “anchor” drug due to the lack of effectiveness of MTX alone. In a meta-analysis of 20 randomized clinical trials, with patients stratified with respect to previous use of disease modifying drugs and with a poor response to monotherapy, MTX monotherapy was more effective in naïve patients than those with insufficient response. That said, this balance of toxicity/effectiveness of monotherapy does not lessen the fact of a greater overall efficacy of MTX in combination with another disease modifying drug.
Should MTX Be Discontinued Before Major Surgery?There is only one report on the use of MTX in the perioperative period of orthopedic surgery. There was no evidence found of an increased incidence of infection compared to the control group. However, no studies exist evaluating the effect of MTX during this period.3
Should Methotrexate Be Discontinued Before Conception?MTX should be discontinued at least 3 months before conception in men and women planning a pregnancy. It should not be used during pregnancy or while breastfeeding.
Six studies have evaluated the use of MTX during pregnancy/lactation with 101 exposed during pregnancy and before conceiving. There were 18 cases of abortions without naming the underlying cause and 5 birth defects. However, no studies have evaluated the use of MTX in men in terms of the rate of abortions/birth defects, in male and female fertility or in breast-feeding newborns. However, expert opinion recommends stopping the drug at least 3 months before a planned pregnancy and avoiding its use during pregnancy and lactation.
Given the above, we conclude that MTX is an effective drug that helps control RA. An analytical and clinical control during its use is important to minimize the risk of a serious complication.
Please, cite this article as: Hernandez-Baldizon S. ¿Cómo hacer buen uso del metotrexato en artritis reumatoide? Reumatol Clin. 2012;8(1):42–45.