Osteoarthritis (OA) is a complex multifactorial disease. The association of knee OA risk with ACE gene rs4343 polymorphism, gene environment synergistic effect, and angiotensin II serum level has not been previously examined. Therefore, we investigate the ACE gene rs4343 polymorphism in knee OA, and its association with severity of knee OA, and angiotensin II serum level.
MethodsUsing a case–control design, we recruited 200 subjects (100 cases and 100 controls) and all were subjected to genotyping of rs4343 SNP by real-time polymerase chain reaction and assay of serum angiotensin II level by ELISA.
ResultsG containing genotypes (AG and GG) and G allele frequencies of the ACE rs4343 polymorphism were significantly higher in the case group than that in the control group. There was significant association between ACE rs4343 genotypes and risk of knee OA under the following genetic inheritance models: GG vs. AA (P=0.003), AA vs. GG/AG (P=0.014), AG/AA vs. GG (P=0.037), and G vs. A (P<0.001). Stratified analyses showed ACE rs4343 polymorphism was evidently associated with a significantly increased risk of knee OA among those had BMI≥25% (adjusted OR=3.016; 95% CI 1.052–8.648; P=0.040). Additionally, knee OA patients with GG genotype had greater knee specific WOMAC index, Kellgren score, and serum angiotensin II level than those with AA or GA genotypes.
ConclusionThe investigated polymorphism in the ACE gene rs4343 may reflect the risk and severity of knee OA in the Egyptian population, particularly with the GG genotype. The interaction between ACE gene rs4343 polymorphism and obesity further increased the risk of knee OA. Moreover, the higher angiotensin II level may be involved in the pathogenesis of knee OA.
La osteoartritis (OA) es una enfermedad multifactorial compleja. La asociación del riesgo de OA de rodilla con el polimorfismo rs4343 del gen ACE, el efecto sinérgico del entorno genético y el nivel sérico de angiotensina II no se ha examinado previamente. Por lo tanto, investigamos el polimorfismo rs4343 del gen ACE en la OA de rodilla y su asociación con la gravedad del OA de rodilla y el nivel sérico de angiotensina II.
MétodosUtilizando un diseño de casos y controles, reclutamos a 200 sujetos (100 casos y 100 controles) y todos fueron sometidos a genotipado del SNP rs4343 mediante reacción en cadena de la polimerasa en tiempo real y análisis del nivel de angiotensina II sérica mediante ELISA.
ResultadosLos genotipos que contienen G (AG y GG) y las frecuencias del alelo G del polimorfismo ACE rs4343 fueron significativamente mayores en el grupo de casos que en el grupo de control. Hubo una asociación significativa entre los genotipos ACE rs4343 y el riesgo de OA de rodilla en los siguientes modelos de herencia genética: GG frente a AA (p=0,003), AA frente a GG/AG (p=0,014), AG/AA frente a GG (p=0,037) y G frente a A (p<0,001). Los análisis estratificados mostraron que el polimorfismo ACE rs4343 se asoció evidentemente con un riesgo significativamente mayor de OA de rodilla entre aquellos que tenían un IMC≥25%. Además, los pacientes con OA de rodilla con genotipo GG tenían un índice WOMAC específico de rodilla, una puntuación de Kellgren y un nivel de angiotensina II sérica mayores que aquellos con genotipos AA o GA.
ConclusiónEl polimorfismo investigado en el gen ACE rs4343 puede reflejar el riesgo y la gravedad de la OA de rodilla en la población egipcia, particularmente con el genotipo GG. La interacción entre el polimorfismo rs4343 del gen ACE y la obesidad aumentó aún más el riesgo de OA de rodilla. Además, el nivel más alto de angiotensina II puede estar involucrado en la patogénesis de la OA de rodilla.
Osteoarthritis (OA) is a chronic degenerative joint disorder1 affects over 250 million people worldwide; most of them are the elderly.2 The fundamental characteristic of this disorder is the gradual progressive damage of articular cartilage, changes in the adjacent bone, and joint space narrowing. It can affect any joint, most commonly in the knees, hips, spine, and hands. Patients with OA typically experience manifestations including chronic joint pain, swelling, stiffness, joint deformities, and limited mobility, which reduce their productivity, lower their quality of life, and increase their social and economic burden.3
Several risk factors associated with knee OA have been proposed, including aging, environmental, and biomechanical factors. Furthermore, genetic factors account for 50% of the risk of OA development and prior research reveals that OA is primarily influenced by genetic risk factors due to common population polymorphisms in numerous genes.4,5 The single nucleotide polymorphism (SNP) is more than 90% of human gene polymorphisms, which is the most common and stable gene variant in the human DNA strands.6
The human angiotensin-converting enzyme (ACE) gene (Gene ID: 1636), is located on chromosome 17q23, has a length of 21kb, and consists of 26 exons and 25 introns.3 The renin–angiotensin–aldosterone system (RAAS primary)’s enzyme, ACE, is produced by the ACE gene.7 It can metabolize the vasodilator bradykinin into inactive fragments and catalytically convert angiotensin I to the potent vasoconstrictor angiotensin II (the main effector peptide of RAS).8 As a result, it plays a crucial role in maintaining blood pressure, electrolyte, and fluid homeostasis.7
However, many studies have demonstrated that the RAS system, which includes the ACE and angiotensin receptors, is expressed locally in the synovial tissue of both humans and animals and may have a role in the development of OA. Additionally, it has been demonstrated that ACE inhibitors have a protective effect against OA in a rat due to their targeting of the local RAS, suppressing the stimulation of the angiotensin II signaling pathway.9,10
Numerous polymorphisms on the ACE gene have been identified by molecular biology studies, including Insertion and/or Deletion (I/D) fragment polymorphisms of the 16th intron, and SNP rs4343 in ACE exon 17. Plasma ACE levels vary with polymorphism,3 and Exon 17's ACE rs4343 polymorphism, had the greatest impact on serum ACE concentration.11
The association between ACE gene polymorphisms and different diseases has become a hot spot in the research field; most studies12–15 focus on the pathogenesis of hypertension, diabetes mellitus, and cardiovascular disorders. Poornima et al. have found that ACE gene polymorphisms are closely associated with type 2 diabetes mellitus and gestational diabetes mellitus.16 Moreover, Narne et al. hypothesized that myocardial infarction and coronary artery disease were significantly influenced by the genetic variant of ACE.17 On the other hand, few studies in the Asian population suggested an association between ACE gene (rs4343 and rs4362 variations, and I28005D) polymorphisms and the risk of knee OA.3,16
To our knowledge, no other study has examined the relationship between SNP rs4343 in the ACE gene and the risk of knee OA. In addition, the variations in genetic susceptibility in different ethnic groups have led us to attempt to validate the association between ACE gene polymorphism (rs4343) and knee OA in another ethnicity. Thus, we conducted the present study to investigate the ACE gene rs4343 polymorphism in primary knee OA and its association with clinical findings, severity of knee OA, and angiotensin II serum level (as a product of ACE) in a cohort of Egyptian individuals.
Subjects and methodsStudy designThe study was designed as a case-control study, and conducted in Al-Zahraa University Hospital, Al-Azhar University, during a period Between September 2021 and March 2022 after approval from the research ethics committee of the faculty of medicine for girls, Cairo, Al-Azhar University (approval number 2021121165). All patients (non-pregnant women and men between 40 and 70 year of age) reporting to outpatient clinics of the authors’ department with complaints of non-traumatic knee pain and those who fit into the clinical criteria of 2010 European League Against Rheumatism (EULAR) for diagnosis of knee osteoarthritis18 were included in this study. For the confirmation of the diagnosis, antero-posterior and lateral views of knee radiographs were taken. Patients were excluded if they had (a) secondary OA, (b) any other pathology affecting knee joint, (c) cardiovascular, renal, hepatic, or malignant disease, (d) were on treatment of OA, or (e) pregnant and lactating women's. All healthy individuals between 40 and 70 years of age with no symptoms and signs of knee OA, or any medical conditions, and has normal knee radiology preferably relatives of the cases and friends were enrolled as controls.
ParticipantsThe recruited subjects (cases and controls) were explained the purpose and relevance of the study and those who volunteered were included in the study after informed consent.
Based on the previous study,3 that showed the frequency of AG genotype of the ACE rs4343 polymorphism were significantly higher in the case group (46.8%) compared to that in the control group (27.8%). A sample size was calculated according to these values using MedCalc® version 12.3.0.0 program “Ostend, Belgium” with 95% confidence interval and power of 80% with α error 5%, produced a minimal sample size of 196 participants, which was enough to find such a difference. However, the number will be increased to 200 participants divided into two equal groups’ knee OA group and normal control group to show appropriate results and to increase the strength of the study. Therefore, after screening based on a sample size calculation, the finally selected sample for the case group was 100 knee OA cases, and the sample for the normal control group was 100 participants to meet our objective.
ProcedureAll participants were subjected to full history taking, thorough clinical examination with stress on musculoskeletal examination, both antero-posterior and lateral views of knee radiographs, as well as laboratory investigations.
Assessment of OA disease activity was done using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). WOMAC is a self-administered questionnaire consisting of 24 items divided into 3 subscales: pain (5 items), stiffness (2 items), and physical function (17 items). The test questions are scored on a scale of 0–4, which correspond to the following: none (0), mild (1), moderate (2), severe (3), and extreme (4). The scores for each subscale are summed up, with a possible score range of 0–20 for pain, 0–8 for stiffness, and 0–68 for physical function. A sum of the scores for all 3 subscales gives a total WOMAC scores.19
In knee OA group, radiological severity was assessed according to a 5-point scale (0–4) Kellgren–Lawrence (K–L) grading system into mild grade (K–L grade 1 or 2), moderate grade (K–L grade 3), and severe grade (KL grade 4) OA. K–L grading of the control group was also done (all grade 0).19 In addition, the body mass index (BMI=body weight (kg)/height square (m2)) was calculated for each participant.20
Laboratory investigationsSample collection and measurementEach participant underwent a clean venipuncture technique to provide 7 milliliters fasting blood sample. The sample was split into two portions; the first portion was collected on two EDTA-containing tubes, the first tube was used for CBC and ESR analysis while the second tube was kept at −20 for DNA extraction and genotyping. The second portion was transferred into a serum separator tube, left to agglutinate for 30min, then centrifuged. The obtained serum was used for CRP measurement and the remaining part was stored at −20°C until measurements of angiotensin II. CRP was done using COBAS INTEGRA 400 Plus autoanalyzer and ESR was estimated by the Westergren method.
Angiotensin II was measured by the ELISA technique. Human Angiotensin II ELISA Kit supplied by SunRed Biotechnology Company with lot No 202107 according to the manufacturer's instructions was used. The assay has an assay range from 105pg/ml to 32pg/ml and a sensitivity of 1.352pg/ml. ELISA reader 1851 Das; Italy and 10641412 Bio Tek; USA washer was used.
Molecular analysis of blood sampleGenomic DNA extractionDeoxy-ribonucleic acid (DNA) was extracted from blood samples using thermo scientific GeneJET Whole Blood Genomic DNA Purification Mini Kit (Lot. No. 00672154) DNA samples were tested for quality and concentration with QIA Expert, and preserved at −20°C. The genotypes of ACE gene polymorphism were performed by RT-PCR (Rotor-Gene from Qiagen) using the TaqMan® SNP Genotyping Assay kit.
ACE (rs4343) genotyping by thermal cycler polymerase chain reactionThe PCR process consists of a series of temperature cycles that are repeated 25–50 times. Each cycle consists of the following steps: initial denaturation at 95°C allows the separation of nucleic acid double chain; annealing at 58°C, allowing binding of the primers, and extension by DNA polymerase at 72°C. The genotyping PCR reaction was performed in a 20μl reaction volume containing. i. TaqMan Genotyping Master Mix 10μl Lot 00726613. ii. TaqMan Genotyping assay Mix 0.5μl. iii. Diluted DNA template 9.5μl. After amplification, data was collected and reading was performed, based on fluorescence signals, using Rotor-Gene real-time system from QIAGEN. Each TaqMan® SNP Genotyping Assay contains 1. Sequence-specific forward and reverses primers to amplify the polymorphic sequence of interest. 2. Two TaqMan® minor groove binder (MGB) probes with nonfluorescent quenchers (NFQ): One VIC™-labeled probe to detect Allele 1 sequence, and One FAM™-labeled probe to detect the Allele 2 sequence. TaqMan® Genotyper Software, the Thermo Fisher Cloud Genotyping Application, and real-time PCR instrument software plot the results of the allelic discrimination data as a plot of Allele 1 (VIC™ dye) versus Allele 2 (FAM™ dye). The allelic discrimination (AD) plot, also known as a cluster plot or scatter plot, represents each sample well as an individual point on the plot.
Statistical analysisThe recorded data were analyzed using the statistical package for social sciences, version 23.0 (SPSS Inc., Chicago, Illinois, USA). Frequency and percentage were used for the qualitative data, and means with standard deviations (SD), and ranges were used for the quantitative data. Independent-samples t-test were used to compare the study groups regarding parametric data while the Chi-square test (χ2) was used in the comparison between two groups with qualitative data. Hardy–Weinberg equilibrium (HWE) for the distributions of genotypes was estimated by the Chi-square (χ2) test. A one-way analysis of variance (ANOVA) test was used to compare more than two groups using quantitative data and a parametric distribution. Odds ratios (ORs) and adjusted ORs (AORs) with 95% confidence intervals (CIs) were calculated to evaluate the associations between ACE rs 4343 polymorphism and the risk of knee OA by logistic regression analyses. Stratifications for Age, sex, and BMI, were subsequently conducted for further analyses. All significant tests were two-tailed and were considered statistically significant at P<0.05.
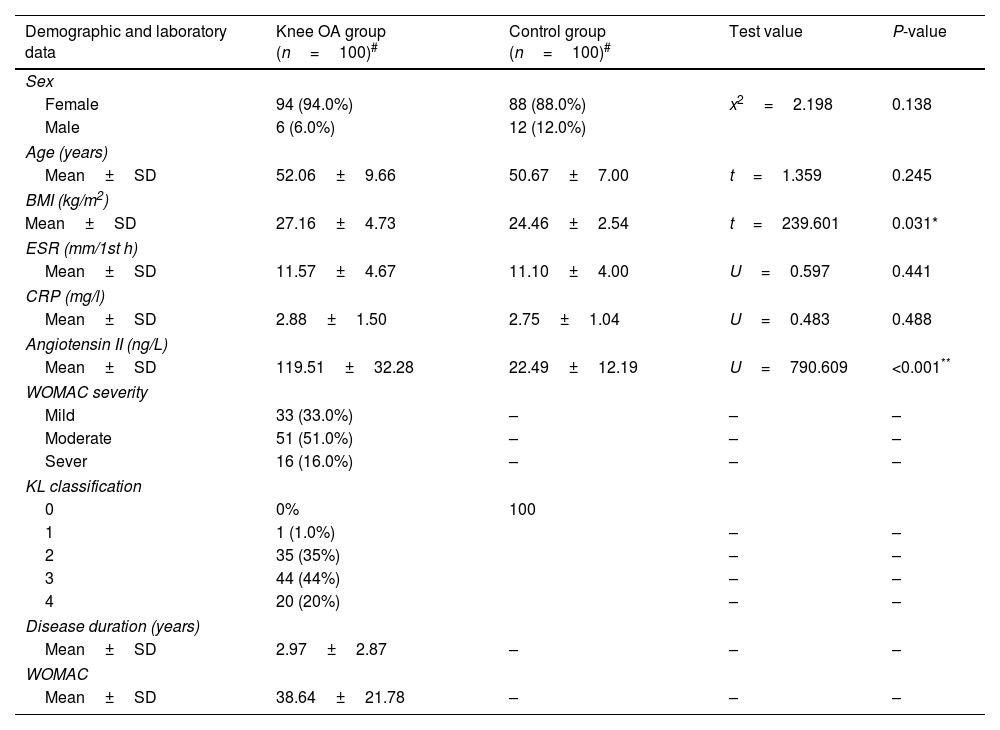
ResultsClinical information of the study subjectsTable 1 presents the demographic, and the clinical data of the population studied as well as the number of individuals in each group. For case group, 94% of knee OA patients were female and the ratio was 6% for males. The ratios of females and males were 88 and 12% in the control group, respectively. There was no significant difference in gender ratio between two groups (P=0.138). The average age of case group and control group were 52.06±9.66 and 50.67±7.00 years respectively, and mean BMIs were 27.16±4.73 and 24.16±2.54kg/m2 respectively. There were no significant differences between the two groups regarding age, sex, ESR, and CRP level. According to the K–L grade, 1% of our patients have grade I, 35% grade II, 44% with grade III, and 20% were classified as grade IV. The Western Ontario and McMaster Universities OA (WOMAC) Index, depends on the assessment of pain, stiffness, and function. 16% of our studied patients have a more severe WOMAC score, as shown in Table 1.
Characteristic of the study subjects.
| Demographic and laboratory data | Knee OA group (n=100)# | Control group (n=100)# | Test value | P-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 94 (94.0%) | 88 (88.0%) | x2=2.198 | 0.138 |
| Male | 6 (6.0%) | 12 (12.0%) | ||
| Age (years) | ||||
| Mean±SD | 52.06±9.66 | 50.67±7.00 | t=1.359 | 0.245 |
| BMI (kg/m2) | ||||
| Mean±SD | 27.16±4.73 | 24.46±2.54 | t=239.601 | 0.031* |
| ESR (mm/1st h) | ||||
| Mean±SD | 11.57±4.67 | 11.10±4.00 | U=0.597 | 0.441 |
| CRP (mg/l) | ||||
| Mean±SD | 2.88±1.50 | 2.75±1.04 | U=0.483 | 0.488 |
| Angiotensin II (ng/L) | ||||
| Mean±SD | 119.51±32.28 | 22.49±12.19 | U=790.609 | <0.001** |
| WOMAC severity | ||||
| Mild | 33 (33.0%) | – | – | – |
| Moderate | 51 (51.0%) | – | – | – |
| Sever | 16 (16.0%) | – | – | – |
| KL classification | ||||
| 0 | 0% | 100 | ||
| 1 | 1 (1.0%) | – | – | |
| 2 | 35 (35%) | – | – | |
| 3 | 44 (44%) | – | – | |
| 4 | 20 (20%) | – | – | |
| Disease duration (years) | ||||
| Mean±SD | 2.97±2.87 | – | – | – |
| WOMAC | ||||
| Mean±SD | 38.64±21.78 | – | – | – |
#Values are mean±SD or the number (percentage), using: t=independent sample t-test; x2: Chi-square test; U=Mann–Whitney test.
Abbreviations: BMI, body mass index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; KL, Kellgren–Lawrence; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; OA, osteoarthritis. P value≥0.05 NS.
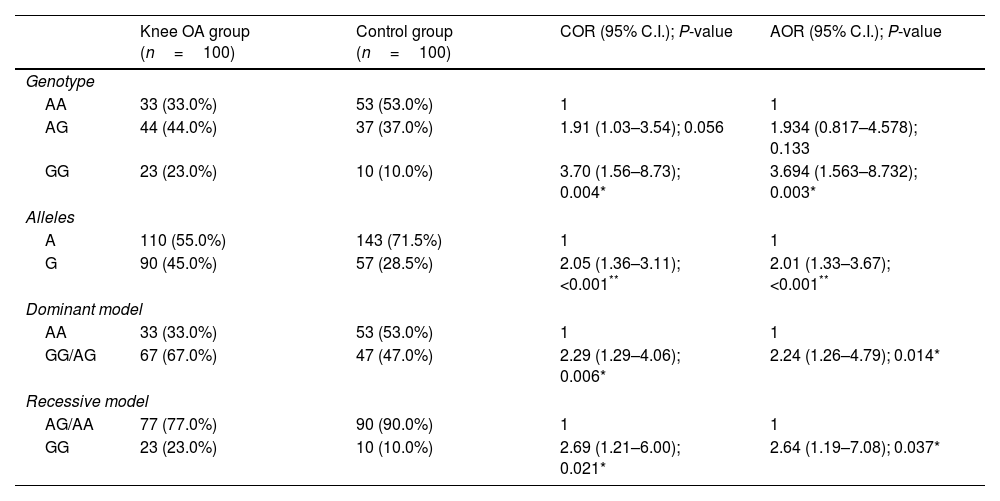
The ACE rs4343 genotype frequency was 23% for GG, 44% for AG, and 33% for AA in the case group; and 10% for GG, 37% for AG, and 53% for AA. The Hardy–Weinberg equilibrium (HWE) test indicated no significant differences in the case group and in the control group (P>0.05). There was significant difference in genotype distribution and allele frequency between case group and control group. The minor G allele was significantly higher in the case group than that in the control group (45 vs. 28.5%, P<0.001, OR=2.05, 95% CI: 1.36–3.11). The allele and genotype frequencies of ACE gene rs4343 polymorphism in the case group and control group are summarized in Table 2.
Associations between ACE gene rs4343 polymorphism and knee OA risk.
| Knee OA group (n=100) | Control group (n=100) | COR (95% C.I.); P-value | AOR (95% C.I.); P-value | |
|---|---|---|---|---|
| Genotype | ||||
| AA | 33 (33.0%) | 53 (53.0%) | 1 | 1 |
| AG | 44 (44.0%) | 37 (37.0%) | 1.91 (1.03–3.54); 0.056 | 1.934 (0.817–4.578); 0.133 |
| GG | 23 (23.0%) | 10 (10.0%) | 3.70 (1.56–8.73); 0.004* | 3.694 (1.563–8.732); 0.003* |
| Alleles | ||||
| A | 110 (55.0%) | 143 (71.5%) | 1 | 1 |
| G | 90 (45.0%) | 57 (28.5%) | 2.05 (1.36–3.11); <0.001** | 2.01 (1.33–3.67); <0.001** |
| Dominant model | ||||
| AA | 33 (33.0%) | 53 (53.0%) | 1 | 1 |
| GG/AG | 67 (67.0%) | 47 (47.0%) | 2.29 (1.29–4.06); 0.006* | 2.24 (1.26–4.79); 0.014* |
| Recessive model | ||||
| AG/AA | 77 (77.0%) | 90 (90.0%) | 1 | 1 |
| GG | 23 (23.0%) | 10 (10.0%) | 2.69 (1.21–6.00); 0.021* | 2.64 (1.19–7.08); 0.037* |
COR, crude odds ratio; AOR, adjusted odds ratio; CI, confidence level. P value≥0.05 NS.
There was significant association between ACE rs4343 genotypes and risk of knee OA under the following genetic inheritance models: GG vs. AA (OR=3.70, 95% CI: 1.56–8.73, P=0.004); AA vs. GG/AG (OR=2.29, 95% CI: 1.29–4.06, P=0.006); AG/AA vs. GG (OR=2.69, 95% CI: 1.21–6.00, P=0.021); and G vs. A (OR=2.05, 95% CI: 1.36–3.11, P<0.001). Moreover, we conducted the multiple logistics to assess the association between ACE rs4343 polymorphism and knee OA risk by adjusting some potential factors including sex, age, and BMI. The results were still significant in each model as shown in Table 2.
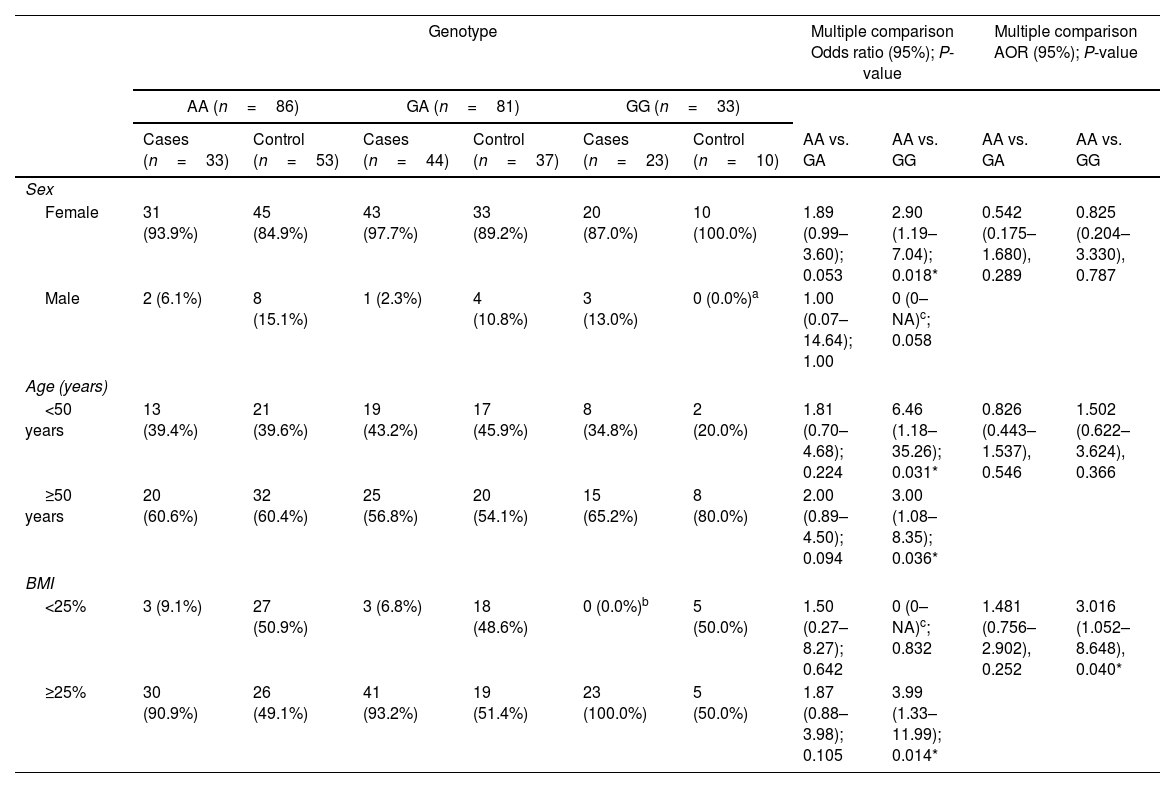
We then further evaluated the effect of ACE r4343 polymorphism on the risk of OA after stratification based on sex, age, and BMI (Table 3). The significant association between ACE r4343 polymorphism and increased risk of knee OA was observed among those who had BMI≥25% after adjusting for age and sex (adjusted OR=3.016; 95% CI 1.052–8.648; P=0.040) (Table 3).
Stratified analysis between ACE gene rs4343 polymorphism and the risk of knee OA.
| Genotype | Multiple comparison Odds ratio (95%); P-value | Multiple comparison AOR (95%); P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA (n=86) | GA (n=81) | GG (n=33) | ||||||||
| Cases (n=33) | Control (n=53) | Cases (n=44) | Control (n=37) | Cases (n=23) | Control (n=10) | AA vs. GA | AA vs. GG | AA vs. GA | AA vs. GG | |
| Sex | ||||||||||
| Female | 31 (93.9%) | 45 (84.9%) | 43 (97.7%) | 33 (89.2%) | 20 (87.0%) | 10 (100.0%) | 1.89 (0.99–3.60); 0.053 | 2.90 (1.19–7.04); 0.018* | 0.542 (0.175–1.680), 0.289 | 0.825 (0.204–3.330), 0.787 |
| Male | 2 (6.1%) | 8 (15.1%) | 1 (2.3%) | 4 (10.8%) | 3 (13.0%) | 0 (0.0%)a | 1.00 (0.07–14.64); 1.00 | 0 (0–NA)c; 0.058 | ||
| Age (years) | ||||||||||
| <50 years | 13 (39.4%) | 21 (39.6%) | 19 (43.2%) | 17 (45.9%) | 8 (34.8%) | 2 (20.0%) | 1.81 (0.70–4.68); 0.224 | 6.46 (1.18–35.26); 0.031* | 0.826 (0.443–1.537), 0.546 | 1.502 (0.622–3.624), 0.366 |
| ≥50 years | 20 (60.6%) | 32 (60.4%) | 25 (56.8%) | 20 (54.1%) | 15 (65.2%) | 8 (80.0%) | 2.00 (0.89–4.50); 0.094 | 3.00 (1.08–8.35); 0.036* | ||
| BMI | ||||||||||
| <25% | 3 (9.1%) | 27 (50.9%) | 3 (6.8%) | 18 (48.6%) | 0 (0.0%)b | 5 (50.0%) | 1.50 (0.27–8.27); 0.642 | 0 (0–NA)c; 0.832 | 1.481 (0.756–2.902), 0.252 | 3.016 (1.052–8.648), 0.040* |
| ≥25% | 30 (90.9%) | 26 (49.1%) | 41 (93.2%) | 19 (51.4%) | 23 (100.0%) | 5 (50.0%) | 1.87 (0.88–3.98); 0.105 | 3.99 (1.33–11.99); 0.014* | ||
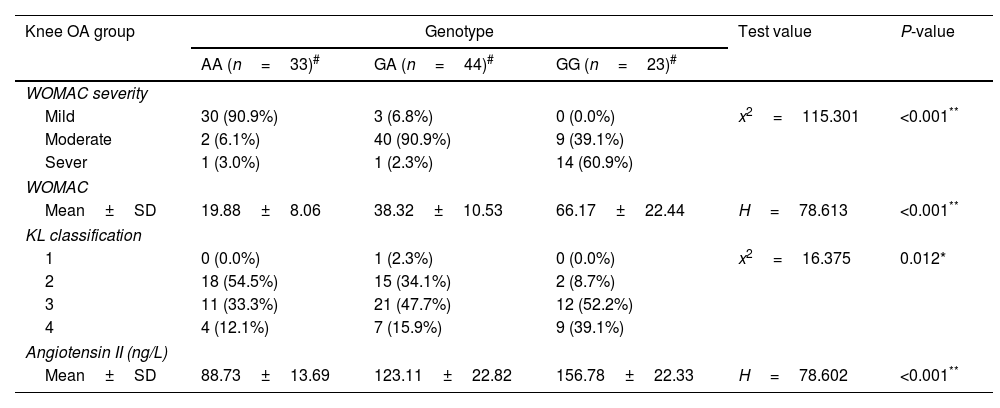
Furthermore, we also, assessed the association between ACE r4343 polymorphism and clinical features of knee OA patients. As shown in Table 4, the results indicated that the GG genotype of rs 4343 polymorphism was associated with higher knee specific WOMAC index and Kellgren score.
The association between ACE rs4343 polymorphism and clinical features of knee OA.
| Knee OA group | Genotype | Test value | P-value | ||
|---|---|---|---|---|---|
| AA (n=33)# | GA (n=44)# | GG (n=23)# | |||
| WOMAC severity | |||||
| Mild | 30 (90.9%) | 3 (6.8%) | 0 (0.0%) | x2=115.301 | <0.001** |
| Moderate | 2 (6.1%) | 40 (90.9%) | 9 (39.1%) | ||
| Sever | 1 (3.0%) | 1 (2.3%) | 14 (60.9%) | ||
| WOMAC | |||||
| Mean±SD | 19.88±8.06 | 38.32±10.53 | 66.17±22.44 | H=78.613 | <0.001** |
| KL classification | |||||
| 1 | 0 (0.0%) | 1 (2.3%) | 0 (0.0%) | x2=16.375 | 0.012* |
| 2 | 18 (54.5%) | 15 (34.1%) | 2 (8.7%) | ||
| 3 | 11 (33.3%) | 21 (47.7%) | 12 (52.2%) | ||
| 4 | 4 (12.1%) | 7 (15.9%) | 9 (39.1%) | ||
| Angiotensin II (ng/L) | |||||
| Mean±SD | 88.73±13.69 | 123.11±22.82 | 156.78±22.33 | H=78.602 | <0.001** |
#Values are mean±SD or the number (percentage), using: H-Kruskal–Wallis test; x2: Chi-square test.
Abbreviations: KL, Kellgren–Lawrence; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; OA, osteoarthritis. P value≥0.05 NS.
The mean serum level of Angiotensin II in knee OA patients was 119.51±32.28ng/L and 22.49±12.19ng/L in healthy controls, (Table 1). The serum Angiotensin II levels were significantly higher in knee OA patients than those in healthy controls (P<0.001); besides patients with ACE rs4343 GG genotype had significantly higher serum angiotensin II level compared to GA or AA genotypes (Table 4).
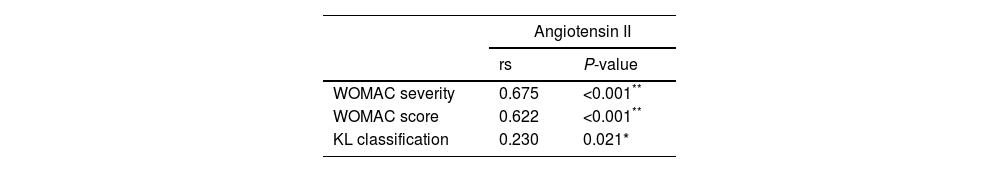
Using Pearson's correlation coefficient, we further analyzed the correlation between angiotensin II level, clinical, and radiographic severity parameters among knee OA patients. The serum angiotensin II levels were positively correlated to the WOMAC severity (r: 0.675, P<0.001), WOMAC score (r: 0.622, P<0.001), and the radiographic severity determined by K–L grading of knee OA (r: 0.230, P=0.021) (Table 5).
DiscussionThe present study found that ACE gene rs4343 polymorphism was associated with knee OA risk. Stratified analysis further indicated significant association among those who had BMI≥25%. Furthermore, the study found GG genotype of rs4343 polymorphism was associated with greater knee specific WOMAC index, Kellgren score, and serum angiotensin II level than those with AA or GA genotypes. Moreover, serum angiotensin II levels were positively correlated to the WOMAC severity, and the radiographic severity of knee OA.
Osteoarthritis (OA) is the most common chronic degenerative and disabling joint disease prevalent in middle-aged and older people worldwide.20 The exact pathogenesis of OA remains largely unclear, but it is believed that genetic polymorphisms greatly contribute to the susceptibility to complex diseases such as OA.3 The most frequent genetic variation in the human DNA chain is known as single nucleotide polymorphism (SNP), which accounts for more than 90% of all human gene polymorphism and is used to identify the genetic variables that are associated with the disease.21 A variety of polymorphisms has been discovered on the angiotensin-converting enzyme (ACE) gene, and these polymorphisms affect both ACE expression and plasma levels.22,23 Even though ACE gene has several polymorphisms, most studies16,17 focus on the insert and/or deletion (I/D) fragment polymorphisms of the 16th intron. On the other hand, only one study in the Asian population suggested an association between ACE gene polymorphisms (rs4343 and rs4362) and the risk of knee OA.3 Recently, it has been shown that the renin–angiotensin system (RAS) is directly involved in bone metabolism, and ACE inhibitors have been shown to have beneficial effects attributed to reduced level of angiotensin II and suppressed stimulation of the angiotensin II signaling pathway.9
Thus, we investigate for the first time in the Egyptian population the ACE gene rs4343 polymorphism in primary knee OA, and its association with clinical findings, severity of knee OA, and angiotensin II serum level.
Genotyping of rs4343 polymorphism in the ACE gene revealed 3 genotypes (GG, AG, and AA). AA genotype and A allele were used as the reference genotype and allele respectively. In the present study, we found that the frequency of GG genotype and G allele of the ACE rs4343 polymorphism were significantly higher in knee OA patients when compared to healthy controls. When compared with the AA genotype, the GG genotype showed 3.6-fold increased risks of developing knee OA. Similarly, the odds of getting OA in G allele carriers were 2.0 times more than those who carry the A allele. In addition, we found that GG genotype greatly increased the risk of OA among those with BMI≥25%. In accordance with our findings, a study in the Asian population done by Qing et al.3 revealed an association between ACE gene rs4343 polymorphism and the risk of knee OA. This study involved 109 knees OA patients and 114 healthy controls, and they found that the ACE gene rs4343 polymorphism was significantly higher in knee OA patients than in the controls, and the G allele might be a risk factor for knee OA.3
Using linkage and association analyses, Zhu et al.11 discovered a significant number of functional polymorphisms in the ACE gene. Recent research suggests that even silent mutations may have functional effects and raise the risk of disease in humans.24 These mutations can affect the folding, stability, and translation rate of mRNA. They may also result in abnormal mRNA splicing. The related protein's expression, structure, secretion, substrate selectivity, and/or enzymatic activity will be impacted by alterations to the mRNA molecule.24,25 Additionally, it has been hypothesized that the ACE gene rs4343 polymorphism modifies the regulatory motifs for several transcription factors, which in turn affects the levels of ACE.26 Moreover, McKenzie et al.27 showed that ACE rs4343 polymorphism could impact the level of ACE expression in serum or tissues and that the G allele might cause a considerable change in ACE expression level. Accordingly, it appears that the ACE rs4343 G allele enhances the ACE expression level and activity, which results in greater levels of angiotensin II.28
Angiotensin II (Ang II) is a physiologically active octapeptide that is produced by ACE-mediated angiotensin I cleavage and is implicated in a variety of inflammatory responses in addition to controlling blood pressure, serum electrolytes, and internal homeostasis in the human body.29,30 By interacting with two different angiotensin receptors, primarily AT1R and AT2R, angiotensin II regulates cell proliferation and apoptosis.29 Angiotensin II can also create a variety of growth factors, including platelet-derived growth factor (PDGF), insulin-like growth factor 1, and vascular endothelial growth factor (VEGF).31–33 Moreover, the production of pro-inflammatory cytokines such IL-6 (Interleukin-6), tumor necrosis factor-alpha (TNF-), and CCL2 (C-C motif chemokine ligand 2) is modulated by angiotensin II, which has been demonstrated in other studies to be crucial for the development and progression of OA. According to earlier studies, Ang II is also thought to reduce osteocalcin mRNA expression and alkaline phosphatase activity. The benefits of RAS inhibition on protecting cartilage could therefore be attributed mostly to lower Ang II levels and downstream signaling in OA.9
In the tibial end of the OA rats’ group, Tang et al.10 discovered that the expression of RAS components such as ACE and the angiotensin 1receptor (AT1R) was significantly enhanced whereas AT2R was decreased. Interestingly, captopril treatment prevented RAS activation and markedly decreased the expression of ACE, AT1R, and Ang II, while increasing AT2R in the captopril-treated OA rat group compared to the untreated OA rat group. In a rat OA model, captopril also has protective effects on cartilage, since it slowed down cartilage degeneration. Silveira et al.,34 who discovered that OA rats had considerably higher levels of ACE expression and that losartan treatment reduced OA symptoms and corrected histological joint changes in osteoarthritic rats, made similar findings. On the other hand, Wang et al.35 showed that the severity of arthritis was considerably reduced after intra-articular injection of rats with the AT2R agonist (CGP42112), indicating that the upregulation of AT2R may be another mechanism that has a therapeutic role in osteoarthritic rats. Therefore, because it targets the local RAS and reduces OA-induced joint and bone damage, therapeutic inhibition of AT1R or ACE (ACE inhibitors) and AT2R agonists may potentially have a protective impact on OA. Based on our results, there was a significant difference in serum angiotensin II levels between knee OA patients and healthy controls. Our data also showed significant differences among the ACE rs4343 AA, AG, and GG genotypes regarding the circulating angiotensin II serum level, where the GG genotype has the highest concentration followed by the AG genotype followed by AA genotype. These data mean that the presence of the G allele, whether in homozygous or heterozygous form, can affect ACE gene expression and serum angiotensin II level, leading to an increase in the risk of knee OA. In accordance with our study, Ghafil et al.36 who reported that the subjects with ACE polymorphism (rs 4343) GG and GA genotypes have higher plasma angiotensin II levels. On the other hand, some other studies have concluded that despite the effect of this polymorphism on serum ACE levels, there is no influence on angiotensin II levels or aldosterone production.37,38
In addition, our results showed a significant difference between ACE rs4343 genotypes regarding severity of disease (GG the high, GA the intermediate, and AA the low), indicating that GG genotype carriers are more prone to have a severe form of the disease. Moreover, a positive correlation of serum angiotensin II levels with WOMAC severity, and KL grades was detected in the present study, This increase of serum angiotensin II level with the increased severity of knee OA could be explained by the fact that, it is secreted in response to inflammation and increased secretion occur with the degree of inflammation. Unfortunately, we were not able to find similar studies regarding the association of ACE rs4343 genotypes and serum angiotensin II with knee OA radiographic severity in any other populations to date to compare them with the results of the current study. Moreover, the reliance on self-reported data may introduce limitations.
There were few studies focused on the role of ACE gene polymorphism in knee OA, also there were paucity of studies in different ethnic groups with the ACE gene rs4343 polymorphism and knee OA. Therefore, it is necessary to confirm these results by conducting further studies of a larger size in Egyptians and in other diverse ethnic populations.
Also, as a result of absence of wide genomic mapping in Arabian Countries, large-scale GWAS (Genome Wide Association Study) combined with WGS (whole genome sequencing) studies, will be very interesting to validate these results. Another possible limitation of the present study is relatively small sample size of the enrolled subjects with unequal genders proportion in the studied group with female predominance; however, this may be explained by the sex differences in the incidence of OA with large sex bias of OA incidence in females.39 Thus, these results should be validated in more well-designed studies considering the relatively limited sample sizes of subgroup analyses.
ConclusionOur results revealed a possible genetic association between ACE gene rs4343 polymorphism and knee OA, and the GG genotype of rs4343 increased the risk of knee OA in Egyptian population. The interaction between ACE gene rs4343 polymorphism and obesity further increased the risk of knee OA. Moreover, the higher serum angiotensin II level may be involved in the pathogenesis of knee OA. However, due to the limitations of our study, further validation studies with larger size participants are needed to confirm our results.
Human rightsAll procedures performed in this study involved human Participants were in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and approved by the Research Ethics Committee of the Faculty of Medicine for Girls, Al-Azhar University, and informed consent was obtained from all individuals for being included in the study.
Ethics committee approvalThe Research Ethics Committee of the faculty of medicine for girls, Cairo, Al-Azhar University reviewed and approved this work (approval number 2021121165, December 2021).
Author's contributionsAll authors have contributed significantly and equally to the design of this work, data acquisition, analysis, and interpretation. In addition to the writing and revising of this manuscript, all authors approved the final version before submission.
Funding statementThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that there is no conflict of interest regarding the publication of this article.