Biological disease-modifying antirheumatic drugs (bDMARD) have improved the clinical course and quality of life of patients with rheumatoid arthritis (RA). However, some patients failed to respond or have an insufficient response to bDMARD early in the course of the treatment.
ObjectivesTo determine the percentage of RA patients who need to switch due to ineffectiveness in the first year of treatment and to identify specific baseline features as possible predictors of switch due to ineffectiveness in the first year of treatment.
Materials and methodsAn observational retrospective study was conducted with patients with RA that started their first bDMARD. Demographic data, disease characteristics, disease activity data scores, laboratory parameters and treatment at baseline were collected. The proportion of patients who failed to respond and who switched to another bDMARD in the first year of treatment was calculated.
ResultsA total of 437 (364 females, 83.3%) patients with RA were included. The majority of these patients started an anti-TNF-α agent (n=315, 72.1%). Forty-eight (11.0%) patients failed to respond to the bDMARD in the first year of treatment. There were significantly more current or former smokers (p=0.030), with a history of depression (p=0.003) and positive for RF at baseline (p=0.014) in the switch group.
In the multivariate analysis, anti-TNF-α agents use (OR 8.3, 95% CI 2.4–28.8, p=0.001), tobacco exposure (OR 2.3, 95% CI 1.1–4.8, p=0.02) and history of depression (OR 3.1, 95% CI 1.3–7.7) seem to predict the need to switch in the first year of treatment due to ineffectiveness.
Discussion and conclusionIn our study, tobacco exposure and depression appear to be modifiable risk factors associated with early switching due to ineffectiveness. Addressing these factors in daily clinical practice is crucial to enhance the overall response to therapy and improve the well-being of patients.
Los fármacos antirreumáticos modificadores de la enfermedad biológicos (FAMEb) han mejorado la evolución clínica y la calidad de vida de los pacientes con artritis reumatoide (AR). No obstante, algunos pacientes no responden adecuadamente o muestran una respuesta insuficiente a los FAMEb en las primeras etapas del tratamiento.
ObjetivosDeterminar el porcentaje de pacientes con AR que necesitan cambiar de FAMEb en el primer año debido a la falta de eficacia, e identificar características específicas en el inicio del tratamiento como posibles predictores del cambio por falta de eficacia en el primer año de tratamiento.
Materiales y métodosEstudio observacional retrospectivo que incluyó pacientes con AR y que iniciaron su primer FAMEb. Se recopilaron datos clínicos y demográficos, así como datos de actividad de la enfermedad, parámetros de laboratorio y tratamiento en el momento de la inclusión. Se calculó la proporción de pacientes que no respondieron y que cambiaron a otro FAMEb en el primer año de tratamiento.
ResultadosSe incluyeron un total de 437 pacientes con AR (364 mujeres, 83,3%). La mayoría de estos pacientes comenzaron un agente anti-TNF-α (n=315, 72,1%). De estos pacientes, 48 (11,0%) no respondieron al FAMEb en el primer año de tratamiento. En el grupo de cambio, hubo significativamente más fumadores actuales o antiguos (p=0,030), con antecedentes de depresión (p=0,003) y positivos para el factor reumatoide (p=0,014).
En el análisis multivariado, el uso de agentes anti-TNF-α (OR 8,3, IC 95% 2,4-28,8, p=0,001), la exposición al tabaco (OR 2,3, IC 95% 1,1-4,8, p=0,02) y antecedentes de depresión (OR 3,1, IC 95% 1,3-7,7) parecen predecir la necesidad de cambiar en el primer año de tratamiento debido a la falta de eficacia.
Discusión y conclusiónEn nuestro estudio, la exposición al tabaco y la depresión parecen ser factores de riesgo modificables asociados con el cambio temprano debido a la falta de eficacia. Abordar estos factores en la práctica clínica diaria es crucial para mejorar la respuesta general al tratamiento y el bienestar de los pacientes.
Biological disease-modifying antirheumatic drugs (bDMARDs) have revolutionized the treatment of chronic inflammatory rheumatic diseases, such as rheumatoid arthritis (RA), over the past two decades.1 These drugs have significantly improved the clinical and functional outcomes of RA, changed the disease course and enhanced the quality of life of patients.
The bDMARDs are indicated for those who responded inadequately to conventional synthetic DMARDs (csDMARD) or experienced side effects with these drugs. They are highly effective, enabling a high percentage of patients to achieve sustained remission or low disease activity.1–4 However, a significant number of patients treated with bDMARD failed to respond adequately or had an insufficient response, some of them within the few first months of treatment. The underlying reasons for these outcomes and the specific characteristics of such patients have yet to be fully investigated and understood.
While many studies have investigated the predictors of effectiveness and persistence in anti-TNF-α therapy, there has been a notable scarcity of studies that have evaluated the causes of early failure of these drugs.5–7 Some studies have primarily focused on the causes of discontinuation of bDMARDs in long-term treatment, however the factors linked to the early failure of bDMARDs in the first year of therapy have been poorly investigated.8–10
Given the limited knowledge in this area and the importance of identifying potentially modifiable risk factors for early bDMARD failure in patients with RA, this study was conducted. The study aimed to determine the percentage of RA patients who need to switch due to ineffectiveness in the first year of treatment and to investigate the risk factors associated with switching during the first year of bDMARD therapy in a cohort of Portuguese patients with RA.
MethodsStudy design and populationA retrospective cohort study was conducted at the Department of Rheumatology of a University Hospital and included patients with RA (according to the 2010 ACR/EULAR criteria).11 All the patients were registered in the Portuguese Rheumatic Diseases Register (Reuma.pt), started their first bDMARD between June 2000 and December 2021 and had a minimum follow-up of 12 months.
Patient selectionPatients aged 18 years or older who were diagnosed with RA according to 2010 ACR/EULAR criteria that started treatment with a bDMARD and were registered in the Reuma.pt database were included. Patients with psychiatric or cognitive disorders that could interfere with data collection and who were physically or psychologically unable to communicate were excluded. Patients with significant missing data and with a follow-up of bDMARD treatment less than a one year were also excluded.
The Guideline for Good Clinical Practice of the International Conference on Harmonization and the ethical principles of the Declaration of Helsinki were followed. All patients signed informed consent and data were anonymized in accordance with the Portuguese Data Protection Law and the General Data Protection Regulation.
Data collectionSociodemographic, clinical characteristics and laboratory parametersData were mainly collected from the Reuma.pt database and additional information was obtained from local medical records. Sociodemographic characteristics at baseline, such as age and gender, along with disease characteristics including age at onset, disease duration, presence of specific manifestations, such as rheumatoid vasculitis, smoking and alcohol drinking habits, history of depression (based on a previous diagnosis by a psychiatrist) and body mass index (BMI) were collected. Details on concomitant immunosuppressive therapies at baseline (systemic corticosteroids and csDMARD), and type of bDMARD administered were also fully detailed. Laboratory parameters including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), presence of rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPA) and anti-nuclear antibodies (ANA) were collected in all patients.
Disease activity measurementsAt baseline, disease activity score for 28 joints with C-reactive protein (DAS-28-CRP),12 Clinical Disease Activity Index (CDAI)13 and Simplified Disease Activity Index (SDAI)14 were collected. Physical function was assessed through the Health Assessment Questionnaire (HAQ).15
Evaluation of switching in the first yearThe patients were reevaluated during the first year of treatment with bDMARD. This included anamnesis, physical examination, laboratory analysis and assessment of disease activity. The number of patients who switched to another bDMARD due to ineffectiveness were collected. Those patients who did not achieve remission or low disease activity16 in the first year of treatment discontinued their current treatment and switched to a different bDMARD. Patients who discontinued treatment in the first year due to adverse events were excluded. Switch and non-switch groups were compared regarding several variables.
Statistical analysisDescriptive statistics for continuous variables with normal distribution were presented with mean and standard deviation. Categorical variables were presented with absolute and relative (percentage) frequencies. Chi-square test, for categorical variables, t-test, for normally distributed continuous variables, and Mann–Whitney U test, for not normally distributed continuous data were conducted. Moreover, a multivariate logistic regression analysis was performed. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A p-value <0.05 was considered statistically significant. Data analysis was performed using IBM SPSS for Windows (version 26, IBM Corporation Software Group, New York, NY, USA).
ResultsA total of 437 (364 females, 83.3%) patients with RA were included. The mean age was 52.4±11.4 years and the disease duration was 11.8±8.8 years. The majority of these patients started an anti-TNF-α agent as first bDMARD (n=315, 72.1%). The remaining patients started rituximab (n=66, 15.1%), tocilizumab (n=51, 11.7%) and abatacept (n=5, 1.1%).
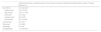
Forty-eight (11.0%) patients failed to respond to the bDMARD in the first year of treatment and needed to switch to another bDMARD. The mean duration of first bDMARD treatment in patients that needed to switch was 0.75±0.3 years. The switching rate was higher among the anti-TNF-α agents, as described in Table 1.
Description of the switching rates due to ineffectiveness in the first year for each biological disease-modifying antirheumatic drug (bDMARD).
| Switching rate due to ineffectiveness in the first year (number of patients that switch/total number of treated patients), % | |
|---|---|
| Anti-TNF-α | 14.3 (45/315) |
| Adalimumab | 9.4 (10/106) |
| Etanercept | 12.9 (17/132) |
| Infliximab | 3.3 (1/30) |
| Certolizumab | 25.0 (2/8) |
| Golimumab | 38.5 (15/39) |
| Tocilizumab | 2.0 (1/51) |
| Rituximab | 3.0 (2/66) |
| Abatacept | 0.0 (0/5) |
| All bDMARDs | 11.0 (48/437) |
Demographic characteristics were similar in the group of patients that switch due to ineffectiveness and patients that did not switch in the first year. Gender, disease duration, age at onset, disease activity scores (DAS-28-CPR, CDAI, SDAI), functional scores (HAQ) and inflammatory parameters at baseline were also similar in the two groups. There were significantly more current or former smokers in the group of patients that needed to switch in the first year of therapy (p=0.030). Moreover, depression was significantly more frequent in the switch group (p=0.003). Positivity for RF at baseline was also significantly more frequent in the switch group (p=0.014).
Regarding the type of bDMARD, patients in the switch group were more frequently treated with anti-TNF-α agents (p<0.001). Table 2 describes demographic and disease characteristics, laboratory parameters, disease activity at baseline and treatment at baseline of the included patients.
Demographic and disease characteristics, laboratory parameters, disease activity at baseline and treatment at baseline of patients with rheumatoid arthritis.
| Switch due to ineffectiveness in the first year(n=48) | No switch in the first year(n=389) | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Gender (female), n (%) | 40 (83.3) | 324 (83.3) | 0.994 |
| BMI, median (IQR) | 26.6 (23.8–31.2) | 26.0 (23.4–29.7) | 0.340 |
| Smoking habits, n (%) | 0.030 | ||
| Former/current smoker | 20 (41.7) | 104 (26.7) | |
| Non smoker | 28 (58.3) | 285 (73.3) | |
| Drinking habits, n (%) | 8 (17.4) | 50 (14.8) | 0.307 |
| Depression, n (%) | 9 (19.1) | 25 (6.7) | 0.003 |
| Disease characteristics | |||
| Age at onset, years, mean±ST | 44.1±11.6 | 42.5±12.7 | 0.413 |
| Disease duration, years, mean±ST | 10.6±7.7 | 11.9±9.0 | 0.337 |
| Presence of vasculitis, n (%) | 2 (4.3) | 3 (0.8) | 0.039 |
| Laboratory parameters at baseline | |||
| ESR, mean±ST | 36.7±25.6 | 38.0±22.3 | 0.72 |
| CRP, mean±ST | 2.0±2.1 | 1.9±2.8 | 0.85 |
| Positivity of RF, n (%) | 44 (91.7) | 296 (76.1) | 0.014 |
| Positivity of ACPA, n (%) | 41 (85.4) | 322 (82.8) | 0.645 |
| Positivity of ANA, n (%) | 17 (35.4) | 132 (33.9) | 0.894 |
| Disease activity at baseline | |||
| CDAI, mean±ST | 31.1±11.6 | 27.8±11.6 | 0.133 |
| SDAI, mean±ST | 33.0±12.5 | 29.7±12.3 | 0.158 |
| DAS-28-CRP, mean±ST | 5.3±1.0 | 5.2±1.2 | 0.42 |
| HAQ, mean±ST | 1.79±0.62 | 1.67±0.64 | 0.249 |
| Treatment at baseline,n(%) | |||
| bDMARD agent | <0.001 | ||
| Anti-TNF-α | 45 (93.7) | 270 (69.4) | |
| Non-anti-TNF-α | 3 (6.3) | 119 (30.6) | |
| Concomitant csDMARD | 42 (87.5) | 320 (82.3) | 0.364 |
| Hydroxychloroquine | 3 (6.3) | 19 (2.3) | |
| Methotrexate | 18 (37.5) | 150 (38.5) | |
| Leflunomide | 11 (22.9) | 108 (27.8) | |
| Sulfasalazine | 4 (8.3) | 6 (1.5) | |
| Methotrexate+sulfasalazine | 6 (12.5) | 37 (9.5) | |
| Concomitant corticotherapyPrednisolone | 41 (85.4) | 348 (89.5) | 0.398 |
ANA: anti-nuclear antibodies; ACPA: anti-citrullinated peptide antibodies; bDMARD: biological disease-modifying antirheumatic drugs; BMI: body mass index: CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IQR: interquartile range; HAQ: Health Assessment Questionnaire; RF: rheumatoid factor; ST: standard deviation.
In the multivariate analysis adjusted for gender and age, anti-TNF-α agents use (versus non-anti-TNF-α agents) (OR 8.3, 95% CI 2.4–28.8, p=0.001), tobacco exposure (OR 2.3, 95% CI 1.1–4.8, p=0.02) and history of depression (OR 3.1, 95% CI 1.3–7.7) seem to predict the need to switch due to ineffectiveness in the first year of treatment, as shown in Table 3.
Multivariate regression model analysis used to identify variables associated with switch due to ineffectiveness in the first year.
| β | OR (95% CI) | p-Value | |
|---|---|---|---|
| Gender (female) | 0.31 | 1.36 (0.53–3.52) | 0.519 |
| Age at first bDMARD | 0.02 | 1.02 (0.99–1.05) | 0.157 |
| Tobacco exposure | 0.84 | 2.32 (1.13–4.75) | 0.022 |
| History of depression | 1.13 | 3.10 (1.25–7.67) | 0.015 |
| Anti-TNF-α agents use (versus non-anti-TNF-α agents) | 2.11 | 8.28 (2.38–28.80) | 0.001 |
| Presence of rheumatoid factor | 0.97 | 2.64 (0.90–7.79) | 0.078 |
| Presence of vasculitis | 1.75 | 5.73 (0.69–47.80) | 0.107 |
bDMARD: biological disease-modifying antirheumatic drugs: TNF: tumor necrosis factor.
In this study, the switching rate due to ineffectiveness was 11.0% for all bDMARDs. Specifically, it was 14.3% (with a range between 3.3% and 38.5% depending on the subtype of anti-TNF-α agent) for anti-TNF-α agents, 2% for tocilizumab and 3% for rituximab. A previous systematic review found a bDMARD discontinuation rate due to ineffectiveness in the first year of 14%, ranging from 10% to 19%.17 Furthermore, a study that analyzed the discontinuation rate of etanercept during the first year of treatment also reported a similar rate of 14.7%.10 In this study, anti-TNF-α agents exhibited a higher discontinuation rate compared to non-anti-TNF-α agents, specifically tocilizumab, rituximab, and abatacept. This finding is consistent with previous literature. Previous studies indicated that abatacept and tocilizumab had lower discontinuation rate due to inefficacy than anti-TNF-α within the first 36 months, with this difference being already observed at 12 months of therapy.18 Additionally, research suggests that patients on abatacept switched less frequently than those on anti-TNF-α in the first 12 months of treatment.19,20 Rituximab also appeared to have lower discontinuation rate due to inefficacy compared to anti-TNF-α, which is consistent with the findings of this study.20,21
In this study, current and former smokers, patients with depression and those with positive RF and history of rheumatoid vasculitis had a higher rate of switching due to ineffectiveness. This analysis identified tobacco exposure, history of depression and the use of anti-TNF-α agents as predictive factors for switching due to ineffectiveness in the first year of treatment.
Seropositivity for RF and ACPA has been linked to a more aggressive and active form of RA.22,23 This increased disease aggressiveness may explain why RF-positive patients more frequently required to switch the treatment within the first year. However, we observed an association only with RF and not with ACPA. Interestingly, Santos-Moreno et al. reported similar findings in their study. They noted that remission was less frequent among RF-positive patients treated with anti-TNF-α agents compared to RF-negative patients, with no differences being reported in the remission rates of ACPA-positive and ACPA-negative patients.24 Other studies have suggested that positive RF and ACPA titers can predict an inadequate response to anti-TNF-α therapy,25,26 whereas others reported inconsistent and contradictory conclusions.27–29
Rheumatoid vasculitis is an uncommon but severe manifestation of RA that represents a more aggressive form of the disease and influences the treatment approach. A previous study found that patients who were refractory to at least three bDMARDs or two bDMARDs with different mechanism of action had a higher prevalence of extra-articular manifestations, including vasculitis.30 This corroborates the findings of this study, supporting the idea that vasculitis can be associated with a higher switch rate of bDMARDs. However, it is important to note that the number of patients with rheumatoid vasculitis in this study is small, and therefore, conclusions draw from these results should be interpreted with caution.
Smoking has long been recognized as one of the most important extrinsic risk factors for the development and severity of RA.31,32 Previous literature suggests that patients treated with anti-TNF-α agents who are smokers tend to experience poorer treatment response and drug survival.33–36 One the other hand, smokers treated with rituximab and tocilizumab did not seem to have a worse response to therapy.37,38
Furthermore, a prior study involving patients with RA who started their first bDMARD revealed a significantly higher rate of switching in the first year of treatment among those with a history of depression.39 Additionally, other study that included patients with RA, psoriatic arthritis and axial spondyloarthritis who received bDMARDs over a 2-year period found that the switching was associated with the use of antidepressant and anxiolytic medications.40
Tobacco exposure and depression appear to be modifiable risk factors associated with early switching due to ineffectiveness in patients with RA. These findings underscore the importance of addressing these factors in daily clinical practice to enhance the overall response to therapy and the well-being of patients.
To optimize treatment outcomes, it is imperative to reinforce the importance of smoking cessation in patients with RA. Healthcare providers should engage patients in discussions about quitting smoking and offer effective interventions and support to facilitate smoking cessation.
Depression, another factor linked to early switching due to ineffectiveness in this study, is often underdiagnosed and undertreated in patients with RA.41 To improve patient outcomes, routine screening for depression should be integrated into daily clinical practice of RA management. Identifying and addressing depression early on can have a positive impact not only on the patient's emotional well-being, but also on their ability to manage their illness and on the treatment response.
Some limitations of this study should be acknowledged. Major limitations are related to the single-center retrospective nature of the study and the extended study duration that may interfered with the outcome. Additionally, the small number of patients treated with abatacept may limit the conclusions about the switching rate due to ineffectiveness of this drug.
To our knowledge, this is the first study that identifies specific baseline features as possible predictors of switching due to ineffectiveness in the first year of treatment in a Portuguese cohort of RA patients.
Conflict of interestsNone to declare.