Rheumatoid arthritis (RA) is a complex systemic joint inflammatory disease with differing manifestations and evolution. A valid, reliable and sensitive assessment procedure that is able to differentiate the inflammatory activity is essential in clinical practice, both in terms of reaching therapeutic decisions and assessing the response to treatment.
The methods currently employed to assess the activity of RA are a combination of clinical parameters, laboratory tests and indicators of the progression of the disease, such as the criteria of the American College of Rheumatology (ACR), the Disease Activity Score (DAS) and the Simplified Disease Activity Index (SDAI).
The emergence of new and more effective therapies obliges us to be more demanding in our therapeutic objectives, and therefore to consider the suitability of the methods that we use to follow our patients’ progress.
La artritis reumatoide (AR) es una enfermedad sistémica inflamatoria articular compleja de diferente presentación y evolución. Una evaluación válida, fiable, sensible y diferenciadora de la actividad inflamatoria es esencial en la práctica clínica para tomar decisiones terapéuticas y valorar la respuesta al tratamiento.
Los métodos empleados actualmente para evaluar la actividad de la AR son una combinación de parámetros clínicos, de laboratorio e indicadores de la actividad de la enfermedad, como los criterios del American College of Rheumatology (ACR), el Disease Activity Score (DAS) y el Simplified Disease Activity Index (SDAI).
La aparición de nuevas terapias más eficaces, nos obliga a ser más exigentes en nuestros objetivos terapéuticos y por tanto, a plantearnos la rentabilidad de los métodos que utilizamos para el seguimiento de nuestros pacientes.
Throughout history, different scales have been proposed to consistently and uniformly evaluate activity in Rheumatoid Arthritis (RA): the core set,1 the Paulus2 criteria and the improvement criteria proposed by the American College of Rheumatology (ACR).
At the beginning of the 1990s, van der Heijde et al.4 carried out a study with the objective of formulating a composite index that quantified the disease. To that end, they classified a cohort of patients with RA into two groups, according to the decision to modify or reduce treatment with disease modifying anti-rheumatic drugs (DMARD). Variables that allowed a better differentiation of the 2 situations related to RA activity and obtained a mathematical formula which quantified clinical activity, giving way to the Disease Activity Score3 (DAS).
The DAS is composed by a measurement of joint pain (Ritchie Index, oscillating between 0 and 78), a swollen joint index in 44 joints (oscillating between 0 and 44), ESR and the evaluation of activity by the patient, on a visual analog scale (0–100mm). There is a modified DAS, the DAS28, based on a 28 painful (PJC) and swollen joint count (SJC), much more useful in daily clinical practice and is recommended by EULAR.
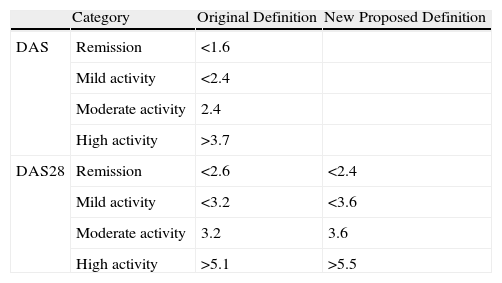
In order to identify patients with different levels of disease activity, numerical limits were set. Cut points separating the three stages were DAS<2.4 for mild activity and DAS>3.7 for high activity, with moderate activity between them.
Van Riel, extrapolated values for DAS28, with DAS28<3.2 for mild activity and DAS28>5.1 for high activity.
Once the different activity levels were defined, Prevoo et al.5 proposed a cut point for the definition of remission of DAS<1.6, using a modification of the ACR criteria of the ACR.
A few years later, the value of DAS was extrapolated to <2.6 (Table 1).
Cut Points for the Activity Categories According to DAS and DAS28.
| Category | Original Definition | New Proposed Definition | |
| DAS | Remission | <1.6 | |
| Mild activity | <2.4 | ||
| Moderate activity | 2.4 | ||
| High activity | >3.7 | ||
| DAS28 | Remission | <2.6 | <2.4 |
| Mild activity | <3.2 | <3.6 | |
| Moderate activity | 3.2 | 3.6 | |
| High activity | >5.1 | >5.5 |
There is no exact definition of the concept of usefulness applied to indexes for the follow up of diseases. We frequently say that disease activity evaluation is useful when the results are close to the reality that we intend to measure with the smallest investment possible.
When questioning the usefulness of DAS28, we should ask two questions: one, if the results approach the real disease activity and two, if the expense, both economical and temporal (time invested) is acceptable.
Real Disease ActivityCorrelation Between the DAS28 Activity and Echography ImagingDuring the past decade questions have arisen regarding the correlation between remission criteria according to DaS and echographic remission, showing an important number of false negatives (patients with remission criteria according to DAS but with activity when explored with echography), especially in mild activity.6,7
Molenaar et al. in 2004, described in patients, with RA in persistent remission, the radiological progression of structural damage, which means that DAS is not capable of detecting low levels of activity which may be clinically undetectable.8
Scire et al.,9 published a study with the objective of evaluating the usefulness of echography in the detection of residual activity in patients with RA, classified as “remission” according to DAS. To that end, a prospective study was performed in 106 patients with RA, which received conventional DMARD, in relation to the activity (DAS) for 24 months. The Doppler signal and the gray scale were correlated with the clinical evaluation and the laboratory data. In clinical remission, 95% of the patients showed residual synovitis and 41% of them had a power Doppler signal, showing that echography can detect residual activity better than the physical examination.
Recently, Balsa et al.10 have published a study with the aim of evaluating the proximity between composite indexes classifying patients in remission, using to that end the absence of activity detected by ultrasound as a gold standard. A total of 97 patients with RA were evaluated, catalogued by their rheumatologist as being in “remission”, using the DAS28 (remission defined as less than 2.6 and the new value of 2.4) and the SDAI (5 and the new value of 3.3). Ultrasound examination was carried out in 42 joints. The presence of synovial hypertrophy was found in 92 (94.8%) and power Doppler in 41 (42.3%) patients. If the absence of power Doppler signal is considered as remission, there were no differences between those that presented remission by DAS28 and those that did not, although differences were found in the SDAI. The results suggested that the SDAI definition of “remission”, was closer to the concept of the absence of inflammatory activity, defined by the absence of a power Doppler signal in the ultrasound.
Therefore, their results provide proof that current methods, such as DAS, are not necessarily indicators of true inflammatory remission and could explain the progression of structural damage, described in patients in clinical remission.
Evaluation and Weight of Data Employed in the DAS 28 FormulaBelmonte11 performed a study in 2008 with the objective of studying the relative weight of each variable in the final result of the DAS 28.
To that end, they tested the value reached by DAS throughout the range of each individual variable, when the rest were set to zero. They observed that the TJC and the ESR provided 35% and 45% respectively, to the value of DAS28. The TJC and global evaluation of the patient contributed 15% only.
Therefore, in spite of the fact that in daily clinical practice we usually give more weight to the SJC than to the TJC, the latter have greater relative weight in the formula.
Regarding the ESR, it is important to emphasize its individual variability and that generated by the different laboratory techniques employed. Another important component is its logarithmic nature, meaning that the lesser the value, the greater its contribution to the formula. Minimal variations, therefore, may produce a leap of almost a whole point in the global index and be the difference between remission and activity or between a good or poor progression, motivating unjustified changes in the therapeutic plan.
It is important to explain the theoretical “floor effect” and the “ceiling effect”. According to its original design, the normal DAS range must oscillate continuously between 1 and 10 points. In clinical practice it is difficult to reach values under 1 or over 9. Therefore, with an ESR of 5mm, the DAS28 is 1.13, with no painful or swollen joints and a patient evaluation of zero.
From a conceptual standpoint, the use of reduced indexes that exclude hips, ankles or feet to evaluate remission has been criticized because they may lead to the cataloguing a patient as being in remission in spite of joint affection.12
In 2007, Kapral et al.13 performed a longitudinal study with 767 patients with Ra, comparing the 32 vs 28 joint counts, excluding in the latter the metatarsophalangeal (MTP) and ankles. In the absence of inflammation, the DAS28 scale had a specificity of 98.1% and a positive predictive value (PPV) 94.1%, and, in the absence of pain, the DAS28 had a specificity and a PPV of 96.1% and 91.7%, respectively. Therefore, the activity index based on the DAS28 may reach levels greater than 2.6 in those patients with feet inflammation because of other values increasing the final result of the index, such as the global evaluation of disease. Therefore, the frequency of remission does not vary when the joint count of 32 becomes 28 in composite indexes.
Invested ResourcesAmong the components of the formula, the only one that involves an economic expense is the measurement of ESR, although it is cheap and easy to perform.14
Even so, it is debatable whether the use of c reactive protein (CRP) would be better to adequately monitor patients with RA. Normally, ESR is not modified as rapidly as CRP, either at the beginning or during the progression of inflammation. In addition, CRP is not as affected as ESR and therefore seems to be a better marker of inflammation.
There are modifications of DAS that use CRP, formulated for their use in clinical trials. The DAS–CRP index has been developed as a mathematical approximation to DAS, making its application and use controversial.
As for the time involved in calculating DAS28, it does not differ greatly from other indexes or test scores employed. For example, 114s are needed to calculate DAS28 compared to 106s needed to calculate CDAI.15
ConclusionsDAS28 has consolidated as a fundamental tool to evaluate RA activity. In contrast with the ACR scores, it is a continuous measurement, lineal, with no need for prior point of reference.
The popularity and the importance of DAS28 are evident not only because it is the currently employed measure in most clinical trials of RA but also because it is included in several clinical practice guidelines for the undertaking of therapeutic decisions.
On the other hand, we cannot omit mentioning that the DAS is an artifice with which we try to ponder a clinically complex construct, such as RA activity. As with all forms of simplification, it has its inconveniences.
DAS 28 activity criteria present a respectable number of false negatives, especially at lower activity scores. It has been shown that some patients who are apparently in remission, radiologic progression may be present, detected through imaging techniques such as echography or MRI.
The weight of each variable in the formula may imply important criteria in the final result, such as the case of ESR or the over evaluation of TJC over SJC.
Therefore, and with the new knowledge available, we must become stricter, working on new tools that allow the evaluation of activity in a more precise, flexible and sensible manner.
However, while a better clinimetric index appears, it would be recommendable to use it as a continuous numerical measure for the evaluation of the intensity of clinical activity in RA.
Note: Section credited by the SEAFORMEC with 1.7 credits. To consult questions of every article in: URL: http://www.reumatologiaclinica.org.
Please cite this article as: Moya Alvarado P, Laiz A. ¿Es rentable la utilización del DAS en el seguimiento clínico de los pacientes con artritis reumatoide? Reumatol Clin. 2011. doi:10.1016/j.reuma.2010.11.018.