Musculoskeletal pain associated to statin use, is the most common adverse event, leading to cessation of treatment. Several studies proposed Vitamin D deficiency to increase the risk of pain associated to statin intake.
ObjectivesTo evaluate whether vitamin D status is linked to musculoskeletal pain associated to statin use.
MethodsWe performed a systematic review based on electronic searches through MEDLINE, Cochrane Central and EMBASE to identify studies that (1) included patients on statin therapy, (2) with vitamin D serum levels assessment, (3) in relation to musculoskeletal pain.
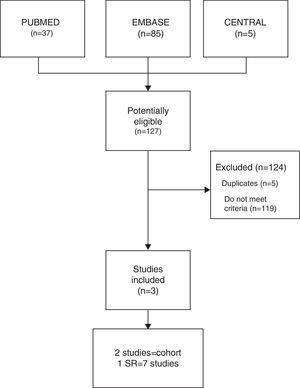
ResultsThe electronic search identified 127 potentially eligible studies, of which three were included and analyzed in the present study. The heterogeneity of studies did not allow metaanalysis. A systematic review and two cohort studies not included in the previous systematic review, revealed a statistically significant association of vitamin D deficit in patients with musculoskeletal pain on statin therapy.
ConclusionThe displayed evidence suggests a significant association between 25OHD serum levels <30ng/ml and the presence of musculoskeletal pain in patients on statin therapy.
El dolor musculoesquelético (DME) asociado a estatinas es el efecto adverso más frecuente y responsable de su abandono. Diversos trabajos sugieren que el déficit de vitamina D incrementa el riesgo de padecer dolor asociado a estatinas.
ObjetivosEvaluar una posible asociación entre el nivel de vitamina D y la presencia de DME en pacientes en tratamiento con estatinas.
MétodosSe realizó una búsqueda bibliográfica en Medline, Cochrane Central y EMBASE para identificar estudios que: 1) incluyeran pacientes tratados con estatinas; 2) en los que valoraran niveles séricos de vitamina D, 3) en relación con DME.
ResultadosSe identificaron 127 estudios de los que se incluyeron y analizaron finalmente 3. La heterogeneidad de los estudios no permitió realizar metaanálisis. Una revisión sistemática y 2 estudios de cohorte no incluidos en la revisión previa mostraron una asociación significativa entre el déficit de vitamina D y el DME.
ConclusionesLa evidencia sugiere una asociación significativa entre niveles séricos de 25OHD <30ng/ml y la presencia de DME.
Statins have demonstrated their efficacy both in the prevention of cardiovascular mortality and its overall reduction.1 As a consequence, the number of patients receiving statin therapy has grown substantially and continues to increase. However, nearly 15%–30% of them will develop musculoskeletal pain (MSP) as the major adverse effect, which often leads to their discontinuing the treatment.2 The mechanism of the production of the pain is unknown and, potential factors include genetic predisposition, a possible mitochondrial dysfunction, a dysfunction involving coenzyme Q synthesis and/or cholesterol).3
Recent studies have suggested that vitamin D deficiency would be associated with MSP induced by statins and, that this could be reversible with vitamin D supplementation and the subsequent normalization of serum 25-hydroxyvitamin d (25OHD) levels.4,5
We performed the present systematic review of the literature for the purpose of determining whether serum 25OHD levels were associated or not with a higher prevalence of MSP related to the intake of statins.
Materials and MethodsSource of Data and Search StrategyA systematic search was performed in 3 databases: Medline, Cochrane Central Register of Controlled Trials (CENTRAL) and EMBASE (up to October 2015), through the documentation service of the Sociedad Española de Reumatología (SER).
Moreover, we performed a manual search of the abstracts from meetings of the American College of Rheumatology (ACR) and the European League Against Rheumatology (EULAR) of the last 3 years. We included studies in English and Spanish.
See the search strategy (Appendix A) (available at the website).
Inclusion Criteria- •
By population: adult patients (≥18years) with any underlying disease being treated with statins (of any type or dose).
- •
By factor: evaluation of serum 25OHD levels.
- •
By outcome: musculoskeletal pain.
- •
By type of study: systemic reviews (SR), cohort and/or longitudinal studies that have been published after the most recently updated SR. Designs that evaluate risk factors (association).
- •
By sample number: >20 per group.
Independently, 2 reviewers (CAP/MBN) reviewed the identified abstracts (inclusion criteria and quality of the selected studies), and differences in criteria were resolved by consensus. The citations were handled using ENDNOTE X, version 7.2.
The quality of the studies was evaluated utilizing New Castle-Ottawa Scale (NOS)6 and the Checklist SIGN (SR). Differences in criteria were resolved by consensus.
Statistical AnalysisWe did not perform a meta-analysis, but did identify a SR2 that utilized weighted mean difference, and used the statistical heterogeneity measured by Cochran Q test and I2.
The results are presented in narrative form.
ResultsThe combined search identified 127 studies, 119 of which were excluded as they did not meet the inclusion criteria, and another 5 were ruled out as they were duplicates. Finally, 3 studies were included (Fig. 1): 1 of which was a SR, by Michalska-Kasiczak et al.,2 that contained 7 studies,1,7–12 as well as, another 2 that were cohort studies, which had been published more recently, Mergenhagen et al.13 and Morioka et al.14
With regard to quality, the SR is acceptable (SIGN), as are the cohort studies (NOS 6–7) (Table 1).
General Features of the Studies.
| Study Country | Studies (included in SR) Country | Study design Registry (years) | Total n MSP (%) | Population Sex (M/W) Age (years) | Statins Doses | MSP 25OHD ng/mL Mean (SD) | Asymptomatic 25OHD ng/mL Mean (SD) |
|---|---|---|---|---|---|---|---|
| MichalsKa-Kasiczak et al., 2015 | Duell and Connor 2008 ND | Cross-sectional ND | n=99 (38.4) | Sex: ND 58–59.3 | All Doses: ND | 20.5 (10) | 30.1 (12.5) |
| Ahmed et al., 2009 United States | Cross-sectional 2007–08 | n=621 (20.6) | MSP=128 (52/76) 58–60 | All Doses: ND | 28.6 (13.2) | 34.2 (13.8) | |
| Linde et al., 2010 United States | Cohort, retrospective | n=64 (60.9) | MSP=39 (21/18) 59 | All Doses: 5–80mg | 28.2 (11.6) | 24.3 (10.5) | |
| Backes et al., 2011 United States | Cohort, retrospective | n=129 (44.2) | MSP=57 (26/31) 58–62.4 | All Doses: ND | 21.4 (9.7) | 21.8 (12.1) | |
| Riphagen et al., 2012 The Netherlands | Prospective, observational 2009–10 | n=75 (29.3) | Sex: ND 65 | All Doses: ND | 18 | 15.2 | |
| Eisen et al., 2014 Israel | Cohort, retrospective 2007–10 | n=272 (39) | MSP=106 (52/54) 66–69 | All Doses: ND | 19.1 (4.1) | 20.2 (6.0) | |
| Palamaner Subash Shanta et al., 2014 United States | Cohort, retrospective 2005–12 | n=1160 (27.8) | Sex: ND 55.9 | Atorvastatin/ Simvastatin Doses: ND | 22.3 (7.1) | 33.8 (4.5) | |
| Mergenhagen et al., 2014 United States | Cohort, retrospective 2006–11 | n=450 (11.1) | MSP=50 (45/5) 65–69 | Simvastatin Doses: 80mg | 26.2 (12.9) | 36.3 (11.8) | |
| Morioka et al., 2015 United States | Cross-sectional, National Health Nutrition Examination Survey (NHNES) 2001–2004 | n=1057 (30.5) | M: 565/W: 492 62.8 | ND | 25OHD <15 OR: 1.90 (1.18–3.05) | 25OHD ≥15 OR: 0.91 (0.71–1.16) |
M, man; MSP, musculoskeletal pain; ND, no data; OR, odds ratio; SD, standard deviation; SR, systematic review; W, woman.
The total population of the studies was 3927 patients, 1038 of whom (26.43%) had MSP, whereas the rest, 2889 (73.53%), were asymptomatic. The mean age of the patients was 61.7years, and ranged between 58 and 69years. There were 1026 women and 1527 men; there were no population-based data from the 3 studies.1,7,12
The drug most widely used was simvastatin2,12,13; it was followed in frequency by atorvastatin,2,12 pravastatina,2,8 and rosuvastatina.1,2 The doses used and the duration of the treatment were specified in only 1 of the cohort studies,13 in which simvastatin was employed at 80mg/day.
With respect to cointerventions, the authors of a single study,9 mention the utilization of other drugs like niacin, fenofibrate, diltiazem and verapamil.
In the SR2 (which included 7 studies; n=2420 patients) 27.5% patients had MSP, probably related to the intake of statins. The comparison of the mean serum levels of 25OHD in patients with MSP and asymptomatic patients, there was a mean of 28.4ng/mL in the first group vs 34.8ng/mL in the asymptomatic group. The weighted mean difference was: −9.41ng/mL (−10.17 to −8.64), with an elevated heterogeneity test (I2=94%; P=.00001).
In the study by Mergenhagen et al.13 (n=450), 11.1% of the patients had MSP. In the comparison of the mean serum 25OHD level in patients with MSP and those who were asymptomatic, there was a mean of 26.2ng/mL in the group with pain vs 36.3ng/mL in the asymptomatic patients, with a mean difference of 10ng/mL (P=.0003).
Finally, in the report by Morioka et al.14 (n=5907), 1057 (18%) of whom received statins, and 30.5% of this group, had MSP. Of these symptomatic patients, 43.8% (33.5%–54.6%) had 25OHD <15ng/mL vs 28% (23.4%–33.2%) who had levels >15ng/mL (P=.01).
These authors calculate the odds ratio (OR) of patients who are taking statins of developing MSP: if the 25OHD level is <15ng/mL, the OR=1.90 (1.18–3.05; P=.01), and if the 25OHD level is >15ng/mL, the OR=0.91 (0.71–1.16; P=.43) (Table 2).
Serum 25OHD Levels in Patients With and Without Musculoskeletal Pain.
| Author/year | Studies included in SR | Population (n) | Musculoskeletal pain n (%) | 25OHD level ng/mL Mean (SD) | P | ||
|---|---|---|---|---|---|---|---|
| With MSP | Without MSP | With MSP | Without MSP | ||||
| Michalska-Kasiczak et al., 2 0 1 5 | Duell and Connor 2008 | 99 | 38 (38.8) | 61 (61.6) | 20.5 (±10) | 30.1 (±12.5) | <.05 |
| Ahmed et al., 2009 | 621 | 128 (20.6) | 493 (79.3) | 28.6 (±13.2) | 34.2 (±13.8) | .0001 | |
| Linde et al., 2010 | 64 | 39 (60.9) | 25 (39.0) | 28.2 (±11.6) | 24.3 (±10.5) | NS | |
| Backes et al., 2011 | 129 | 57 (44.1) | 72 (55.8) | 21.4 (±9.7) | 21.8 (±12.1) | NS | |
| Riphagen et al., 2012 | 75 | 22 (29.3) | 53 (70.6) | 18.0 | 15.2 | NS | |
| Palamaner Subash Shanta et al., 2014 | 1160 | 276 (24.2) | 864 (75.7) | 22.3 (±7.1) | 33.8 (±4.5) | .01 | |
| Eisen et al., 2014 | 272 | 106 (38.9) | 166 (61.0) | 19.1 (±4.1) | 20.2 (±6) | NS | |
| Mergenhagen et al., 2014 | 450 | 50 (11.1) | 400 (88.9) | 26.2 (±12.9) | 36 (±11.8) | .0003 | |
| Morioka et al., 2015 | 1057 | 322.3 (30.4) | 734.7 (69.5) | 25OHD (ng/mL) with MSP | |||
| <15 (ng/mL) 43.8% (33.5–54.6) | >15 (ng/mL) 28% (23.4–33.2) | .01 | |||||
| n Total | 3927 (100%) | 1038 (26.5%) | 2889 (73.5%) | ||||
MSP, musculoskeletal pain; NS, not significant; SD, standard deviation; SR, systematic review.
The present systemic review suggests the existence of an association between vitamin D deficiency and MSP. Therefore, about 1 out of every 4 patients who were receiving statins in the present work, will manifest MSP. In this respect, the definition of MSP related to statins is somewhat confusing and quite vague,15 as there is no internationally standardized classification.16 Its clinical form encompasses, from simple myalgia or muscle weakness, and even rhabdomyolysis. Thus, its most frequently observed presentation is muscle pain that is not associated with an elevation in creatine phosphokinase.16,17 Likewise, it is well-known that reduced serum vitamin D levels are also the cause of generalized MSP,18 and possibly, the presence of vitamin D receptor (VDR) in muscle cells supports this principle.19 The relationship between serum 25OHD levels <30ng/mL and MSP associated with statins has been reported in certain uncontrolled series.8,20 Although, in a speculative manner, it has been estimated that the mechanism of interaction between MSP and statins and vitamin D deficiency could have a relationship with the activation of cytochromes CYP3A4, CYP2B6 and CYP2C9 in hepatocytes.3 Thus, vitamin D is an activator of these cytochromes, which, in turn, are responsible for metabolization of different statins, especially the lipophilic type (atorvastatin, simvastatin, lovastatin, fluvastatin and pravastatin).3,21 Their deficiency could explain a prolongation of the half-life of these drugs and their possible toxicity, resulting in MSP. The muscle fibers affected would be type II, which are also implicated in the myalgia caused by alcohol.22 Moreover, it is suggested that low vitamin D levels could reduce the transcriptional gene linked to VDR, decreasing the synthesis of proteins to repair the t-tubular system and prevent the subsarcolemmal rupture of those fibers.22 The combination of these 2 causal factors, the vitamin D deficiency and statins, would potentiate the involvement in type II muscle fibers, favoring the development of pain. This symptomatology appears to extend even to patients who receive statins on alternate days. Thus, the study of Minissian et al.23 demonstrated that patients with MSP caused by statins, who also received treatment on alternate days for intolerance to these drugs, had significantly decreased serum vitamin D levels when compared with those patients who were tolerant to the daily therapies. In contrast, studies like those of Eisen et al.,11 Kurnik et al.,24 and Backes et al.,10 found no connection among serum vitamin D levels and risk of MSP in patients receiving statins. Possibly, this divergence corresponds to differences in the type of population studied, ethnic groups, type and dose of statins employed or the nutritional status of the patients, etc. Once again, in none of these studies is the season of the year mentioned or are the methods used to assess serum 25OHD levels. In this respect, it is important to point out that vitamin D level deficiency (<30ng/mL or 75nmol/L)25,26 is a prevalent condition in different populations and regions of the world.27–29 Moreover, this situation is becoming more extended and more profound in recent years.30 For this reason, it is appropriate to mention, although it is not within the scope of this analysis, that uncontrolled studies have evaluated the response of patients with MSP due to statins to vitamin D supplementation, with encouraging results.4,5,8
This review has certain limitations: limitations inherent in the type of design and the biases of the studies included (retrospective designs, duration of the registry of inaccurate data, etc.). Likewise, there is little information concerning the types, doses and duration of statin therapy on the part of the patients, and too few references on the presence of comorbidities and/or cointerventions. One particularity to be taken into account is the updated definition of statin-induced MSP, which, in our opinion, is very vague and can lead to confusion. Finally, we understand that it is very important to define the methodology for measuring vitamin D, as well as the season of the year in which the sample is collected and is analyzed, and its evaluation according to age group.
We can conclude that the available evidence (a systematic review and 2 cohort studies) shows a prevalence of MSP associated with statins that ranges between 11% and 30%. There is also a significant association between decreased serum 25OHD levels, and a higher prevalence of statin-related MSP.
Well-designed, long-term, prospective studies are needed to confirm these results.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestThe authors declare they have no conflict of interest.
The authors thank Dr. Loreto Carmona for her collaboration in the review of this article, and Ms. Mercedes Guerra, manager of documents and archives of the Research Unit of the Sociedad Española de Reumatología (SER), for her help in the literature search strategy and her assistance in obtaining the articles.
Please cite this article as: Pereda CA, Nishishinya MB. ¿Existe relación entre los niveles séricos de vitamina D (25OHD) y el dolor musculoesquelético relacionado con la ingesta de estatinas? Revisión sistemática. Reumatol Clin. 2016;12:331–335.