Large granular lymphocyte leukemia is a rare entity belonging to same spectrum of diseases than Felty's syndrome, which might occur in patients with long-standing rheumatoid arthritis. It is clinically characterized by persistent neutropenia and recurrent bacterial infections associated with the presence in both peripheral blood and bone marrow of clonal expansion of atypic lymphocytes with a cytotoxic T cell phenotype, or less frequently an NK-cell phenotype, as well as splenomegaly. It is more frequently diagnosed in seropositive rheumatoid arthritis, with significant structural damage, extra-articular manifestations and persistently elevated values of ESR, despite them having low inflammatory joint activity. We report the case of a 70-year-old male with a long-standing rheumatoid arthritis, who developed septic shock secondary to prosthetic hip infection by Salmonella spp. He showed persistent neutropenia, and an aberrant monoclonal T cell population was detected in both peripheral blood and bone marrow, consistent with large granular lymphocyte leukemia.
La leucemia de linfocitos grandes granulares es una entidad poco frecuente, perteneciente al mismo espectro de trastornos que el síndrome de Felty que puede presentarse en pacientes con artritis reumatoide de larga evolución. Clínicamente se caracteriza por neutropenia persistente e incremento de la susceptibilidad a infecciones bacterianas, asociado a la presencia en sangre periférica y médula ósea de una expansión clonal de linfocitos atípicos con fenotipo de linfocito T citotóxico, o menos frecuentemente de célula NK; y esplenomegalia. Se diagnostica con mayor frecuencia en pacientes con artritis reumatoide seropositiva con importante daño estructural, manifestaciones extra-articulares y valores persistentemente altos de factor reumatoide y VSG a pesar de poder presentar escasa actividad inflamatoria articular. Presentamos el caso de un varón de 70 años con artritis reumatoide de larga evolución que desarrolló shock séptico secundario a la infección de una prótesis de cadera por Salmonella spp. Presentaba neutropenia persistente identificándose en sangre periférica y médula ósea una población monoclonal de linfocitos T aberrantes compatibles con leucemia de linfocitos grandes granulares.
Felty's syndrome (FS) is a rare systemic complication (less than 1%) of rheumatoid arthritis (RA), characterized by the triad of RA, persistent neutropenia (<2000/mm3) and splenomegaly of varying size which can range from subclinical splenomegaly, only detectable by imaging, to massive1 splenomegaly. It occurs mainly in long-standing cases with severe joint disease and extraarticular manifestations, and has a strong association with the HLA-DR4 haplotype (almost 95% of cases).2 In 30%–40% of patients with FS there is an expansion of large granular lymphocytes (LGL).1 LGL represent 10%–15% of circulating mononuclear cells and are morphologically identified by their large size (15–18μm), their round or indented nucleus and abundant cytoplasm with azurophilic granules. The phenotype of these cells may be cytotoxic T lymphocyte (CD8+, CD57+) or natural killer (NK) (CD3−, CD8−, CD56+).3 When expansion of LGL is monoclonal and is associated with infiltration of the bone marrow and spleen by these cells, it is called large granular lymphocyte leukemia (LGLL) and is considered a chronic low-grade lymphoproliferative disorder. Its clinical presentation is similar to that of FS, highlighting the increased susceptibility to bacterial infections associated with neutropenia, anemia and splenomegaly, as has also been called “pseudo-Felty”.3,4
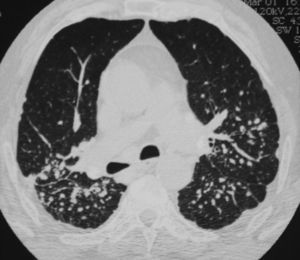
Clinical PresentationA 70-year-old retired worker from a granite quarry was diagnosed at age 43 with seropositive RA with involvement of hands, feet, knees and hips. He subsequently developed pneumoconiosis and pulmonary rheumatoid nodules (Fig. 1), being diagnosed as Caplan's syndrome. During his progression, he was treated with NSAIDs, corticosteroids, gold salts, cyclosporine and methotrexate. Despite this, the patient developed structural damage in the hands, feet and hips, requiring placement of prosthesis in both hips at 51 and 54 years of age, respectively. In recent years, his disease remained stable, treated with methotrexate 10mg weekly and low doses of glucocorticoids, with no evidence of inflammatory joint activity. He presented ‘gooseneck’ deformities in all fingers and rheumatoid nodules on the elbows. 12 months ago he suddenly developed fever and pain in his right groin, with subsequent septic shock, demonstrating an infection of the right hip by Salmonella spp. Methotrexate and glucocorticoids were suspended, and he was treated with prolonged antibiotic therapy and partial replacement of the prosthesis. Laboratory tests evidenced persistent neutropenia, despite the withdrawal of myelotoxic drugs and the improvement of sepsis, reaching 0neutrophils/mm3. Retrospectively, when reviewing the numbers of neutrophils, he had low counts a year earlier, between 1800 and 1000/mm3. The rest of the blood count and biochemistry were normal. The ESR was 80mm/h and CRP 111mg/l. He maintained high levels of rheumatoid factor (6930U/ml) and anti-CCP (300U/ml) and also had polyclonal hypergammaglobulinemia. Antinuclear antibodies and extractable nuclear antigens were negative and complement levels were within normal values. HLA typing showed that he carried the haplotype DRB1*0404 (DR4) and mild splenomegaly was detected (13.7cm) on abdominal computed tomography.
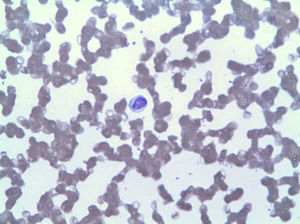
The peripheral blood smear showed lymphocytosis by LGL (Fig. 2), which in the immunophenotype corresponded to 42% of total leukocytes, with an aberrant phenotype of cytotoxic T lymphocyte (CD3+, CD8+, CD5+, CD7+/−, CD4−, CD56− and DR+). The bone marrow biopsy showed 20% of the total marrow cellularity corresponding to the same clonal expansion (confirmed by rearrangement of the variable region of TCR gamma). Based on these findings, a diagnosis of LGLL was made and treatment with methotrexate 15mg weekly restarted despite 3 months with persisting neutropenia (<500/mm3), requiring frequent administration of granulopoiesis stimulatory factors. He was subsequently treated with cyclophosphamide, vincristine and prednisone at high doses. After 6 months of treatment, neutropenia persists.
DiscussionLGLL is a chronic leukemia characterized by expansion of the LGL monoclonal phenotype of activated cytotoxic T lymphocytes or less frequently NK cells.5 The average age of diagnosis is 60 years and it is frequently associated with autoimmune diseases, particularly RA, but has also been described in ulcerative colitis, Sjögren's syndrome, lupus erythematosus and multiple1 sclerosis. Patients with RA who have associated LGLL have a clinical presentation similar to that of FS. They are usually patients with longstanding RA, severe joint damage and significant consequences, and increased frequency of extra-articular manifestations such as rheumatoid nodules, lymphadenopathy, pretibial ulcers, pleuritis, skin pigmentation, neuropathy or episcleritis.6 The patient had also another rare complication of RA, rheumatoid pneumoconiosis or Caplan's syndrome, characterized by the appearance of pulmonary nodules with histopathology similar to that of typical rheumatoid nodules in patients with a history of occupational exposure to inorganic dusts such as silica, coal or granite.7 To our knowledge, the present case is the first describing the presentation of Caplan's syndrome and LGLL in the same patient. In most cases, the presentation LGLL is severe neutropenia associated with recurrent bacterial infections. The microorganisms most commonly involved are Staphylococcus aureus, Streptococcus spp. and gram-negative bacilli. Less commonly anemia, fever, night sweats and liver and spleen enlargement5 may also accompany it. Up to one third of patients with LLGG have no apparent RA clinical activity at the time of diagnosis, but maintain high levels of ESR.6 Up to 40% of patients with FS have LGL lymphocytosis.7 This fact, together with the clinical similarity and association with HLA-DR4 has strongly suggested that FS and LGLL associated with RA are expressions of the same entity characterized by the proliferation of LGL.8 Other forms include also milder forms such as reactive lymphocytosis and infections to more aggressive forms of NK5 LGLL. LGLL diagnosis is based on the finding of a monoclonal expansion of LGL in peripheral blood and bone marrow with a characteristic immunophenotype (CD3+, CD4−, CD8 +, CD16+, CD28− and CD57+). The clonality is confirmed by studying the reTCR9 gene. In general, LGL has a chronic and indolent progression, with a mean survival of 10 years.1 In rare cases, especially when the expansion is due to LGL with NK phenotype, this leukemia can behave more agressively.5 The most common indication for treatment are recurrent infections and, less frequently, anemia, symptomatic splenomegaly or the appearance of severe B symptoms B1.
The first-line treatment in LGLL are immunosuppressive drugs alone, specifically methotrexate (10mg/week), cyclosporin A (1–1.5mg/kg/2 times daily) or oral cyclophosphamide (50–100mg/day). This treatment is effective in about 50% of patients, achieving the correction of cytopenias, but not eradicating leukemic cells.1 Glucocorticoids can be used to speed the response and granulopoiesis stimulating factors are useful in the initial management of neutropenia. In refractory patients and in those with very aggressive presentation treatment with chemotherapy regimens similar to CHOP (cyclophosphamide, vincristine, doxorubicin and prednisone) and other schemes for lymphoma have been tried, but have not clearly demonstrated their effectiveness. Other treatments that have been tested are purine analogues, Alemtuzumab, bortezomib, splenectomy and allogeneic bone marrow transplantation, with variable results.3
ConclusionsBoth FS and LGLL are rare complications of RA, which appear in long-standing disease, with significant structural damage and extra-articular manifestations. In patients with longstanding RA and neutropenia the presence of clonal proliferations of LGL in peripheral blood and/or bone marrow should be ruled out, allowing for the diagnosis of LGLL. First line treatment is the use of immunosuppressive drugs, such as low-dose methotrexate, and glucocorticoids may be associated with granulopoiesis stimulating factors. Other treatment modalities such as chemotherapy or splenectomy have shown variable results in some refractory cases.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Lois Iglesias A, et al. Leucemia de linfocitos grandes granulares como complicación de artritis reumatoide. Reumatol Clin. 2012;8:365–7.