We herein describe an inter-specialists unit for the monitoring and management of biological therapies and analyze the utilization of biological agents across specialties and diseases. Protocols and therapeutic objectives, as well as outcomes and protocol deviations, are shared and discussed periodically between specialists. All patients treated at one centre with any biological treatment from January 2000 by rheumatology, gastroenterology, dermatology, or neurology, regardless diagnosis, are identified by Clinical Pharmacy and included in an ongoing database that detects use and outcome. The drugs, survival, and reasons for discontinuation differ significantly across specialties. This approach has helped us recognizing the challenges and size of the problem of sharing expensive medications across specialties, and has served as a starting point to contribute to the better use of these compounds.
A continuación describimos una unidad interservicios para el seguimiento y la gestión de las terapias biológicas, y analizamos la utilización de los agentes biológicos en las distintas especialidades y enfermedades. Los protocolos y los objetivos terapéuticos, así como los resultados y las desviaciones de los protocolos, se comparten y debaten periódicamente entre los especialistas. Todos los pacientes tratados en el centro con cualquier terapia biológica desde enero de 2000 por reumatología, gastroenterología, dermatología o neurología, independientemente del diagnóstico, son identificados por Farmacia Clínica e incluidos en una base de datos continua, que detecta el uso y los resultados. Los medicamentos, la supervivencia y las razones de interrupción del tratamiento difieren significativamente entre especialidades. Este enfoque nos ha ayudado a reconocer los retos y la magnitud del problema de compartir medicamentos costosos entre especialidades, y ha servido como punto de partida para contribuir a un mejor uso de los mismos.
The discovery and development of biological therapies (BT) is one of the most significant changes in the last two decades in various medical specialties.1–4 Notwithstanding all approved biological agents have proven effective in their various indications,5–8 compared effectiveness and safety cannot be established in the absence of direct comparisons; in addition, much of the evidence on BT safety profiles remains limited and partially related to disease characteristics.9–11 Variability in the use of BT may be related issues, like conflictive clinical information, pharmacology, differences among specialties or patient characteristics or preferences.12,13 Variability, however, may be unacceptable when it is caused by limited physician skills or institutional constraints. The high cost of these treatments explains the need for increased rigour in the selection and monitoring of patients who receive them14–16 and the importance of a management based on equity, efficiency, quality and feasibility.
Based on a common goal of improving management, five specialties in our hospital—namely rheumatology, dermatology, neurology, gastroenterology, and clinical pharmacy—under the auspices of the medical direction, decided to share efforts. Herein we will describe the rational, goals and procedures of the multi-specialist BT unit (BTU) created. In addition, we analyze the past and current situation of the use of biologic agents in the different specialties in our centre, as a starting point to contribute to the better use of these compounds.
MethodsHospital La Princesa and areaLa Princesa is a 564-bed public centre that serves, as per 2013 web report, an area of 310,464 inhabitants in a middle-upper class district of Madrid, Spain. It covers all specialties, except for paediatrics and obstetrics, in a teaching setting with a considerable trajectory of clinical and basic research.
La Princesa Biological Therapies Unit (BTU)Because one of the main goals of this article is to describe the unit, we include its description under the results section.
Benchmarking analysisPrior to the development of the register, the unit members decided upon the conduct of a state analysis that would provide not only data on the use of biological agents at the centre, but also feed-back on the feasibility of merging databases from different departments. The state analysis was approached as an observational retrospective descriptive study. All patients treated at the centre with any biological treatment from January 2000 to December 2012, regardless diagnosis, were identified in the Clinical Pharmacy databases. Only patients followed at the participant departments were included—namely rheumatology, gastroenterology, dermatology, and neurology—as in other departments the biological treatments were anecdotal or related to complications in patients attended at the former ones. Although off-label—not listed as an indication in the summary of product characteristics—uses are included in the analyses, for the purpose of comparison across diseases, we studied the following indications: rheumatoid arthritis (RA), psoriatic arthritis (PsA), psoriasis, multiple sclerosis (MS), Crohn's disease (CD), ulcerative colitis (UC), and ankylosing spondylitis (AS). One must bear into account that an off-label use in any given year may become an accepted indication later on.
For the purpose of this study, we considered as BT the following: etanercept (ETN), infliximab (IFX), adalimumab (ADA), golimumab (GOL), certolizumab (CZL), abatacept (ABA), glatiramer acetate (GLA), anakinra (ANK), efalizumab (EFZ), fingolimod (FGL), interferon beta, 1a and 1b (IFN), natalizumab (NTZ), rituximab (RTX), tocilizumab (TCZ) and ustekinumab (UST).
The variables used for description were: (a) use patterns—indication or condition for which it was stated to be used, off-label or un-specified uses; (b) drug survival, based on dates of start and end of treatments, and whether treatment was maintained at the time of the last visit; (c) reasons for drug discontinuation, such as adverse events, ineffectiveness (discontinuation occurred within 6 months from start), loss of effectiveness (discontinuation occurred after 6 months from start), remission, or other; (d) patient variables, such as age at start of treatment, disease duration from symptoms onset; (e) and study periods, determined by the dispensation date at the beginning of biological treatment (2000–2005, 2006–2010, 2010–2012)—this year division was based on drug availability; of note, efalizumab was withdrawn from the market in 2009 due to the risk of progressive multifocal leukoencephalopathy. Three databases from the Pharmacy department related to drug dispensation, specialty, and cost—inpatient, ambulatory, and biological-specific—were merged with the centre's administrative database.
Statistical analysisFor the statistical analysis, we first carried out a description of the sample (patients and treatments), and by different time periods. Drugs survival was estimated by Kaplan–Meier curves. The retention time of biologic therapy was estimated for all compounds, despite one patient might have received different drugs or different treatments. Analyses were performed by specialty, biological agent, and study period using the appropriate statistical hypothesis contrast test depending on the variables analyzed (chi-square, log-rank, or analysis of variance). In addition, we carried out a multivariate analysis with Cox regression models to compare the influence of agent, disease, and first-line treatment versus successive treatments, in the discontinuation of treatment, either by any cause, by ineffectiveness or loss of effectiveness, or by adverse event. FGL and off-label uses were excluded in this analysis due to the small sample size. The analysis was performed with Stata v.12 (College Station, Texas).
ResultsThe BTU: description, goals and proceduresThe BTU was established in 2012 through a joint effort of professionals with extensive experience and interest in the use of BT from the departments of rheumatology, dermatology, gastroenterology, neurology, pharmacy, and hospital information and technology. The BTU aspires to contribute to the better use of BT, targeting the balance between sustainability and a favourable risk/benefit ratio for patients. Three major objectives were established: (1) Contribute to increasing the quality of use of biologics; (2) Contribute to sustainability of the use of BT; (3) Generate information for audits and publications. (1) The first objective includes issues like developing and updating protocols for the use of BT in different conditions, defining the therapeutic goal for our patients on BT, and defining quality indicators. These include: Percentage of patients with an indication for BT, patients with objective measures of disease activity recorded in clinical chart, patients meeting the therapeutic goal, and patients with BT withdrawn due to lack of efficacy. (2) The second objective includes initiatives to favour cost-effectivity. It includes measures like: favouring the use of drugs with lower cost, unless the existence of medical reasons for not doing so, regularly updating the information on the cost of BT to prescriptors, grouping patients under the same drug that is dosed by weight on the same day, in order to limit drug waste, or favouring the judicious use of biosimilars and innovative ways of optimization, like risk-sharing agreements. Indicators in this area include cost per patient, patients with dose of BT increased (intensifications), patients with dose of BT decreased (optimizations) or percent use of biosimilars. (3) The third objective includes two major goals: (a) an observational retrospective analysis of the use of BT in our hospital whose major results are included in this article and (b) the development of a prospective register enabling the conduct of clinical studies and practice audits.
The BTU is formed by one coordinator, one or two members of the different services involved and one full time data analyst. They hold periodic meetings to define objectives, share the results of analyses, discuss studies in their respective fields that can be of interest to others, as well as difficulties or challenges. It is important to clarify that the BTU does not decide at the individual level which biologic will be used for each patient. This decision still corresponds to the practicing specialist and each of the departments establishes its own way of deciding. In the case of rheumatology, two weekly sessions are organized for making these decisions.
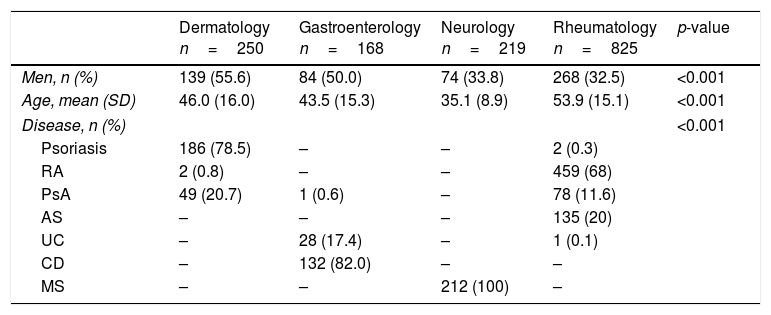
Use of biologic therapies at our hospitalThe databases contained information on 1465 patients and 2238 treatments through the study period. Table 1 shows a description of the patients included, by specialty. The mean age of the patients as of first biological treatment was 48±16 (SD), and 61% were women. The patients treated with biologics in the four specialties were different in age, percentage of sex, and, of course in diseases, with RA (35.9%) as most frequent disease, followed by MS (16.5%), and psoriasis (14.6%).
Patients receiving any dose of any biological therapy at the centre in the study period. Description at the time of the first agent utilized, by specialty.
| Dermatology n=250 | Gastroenterology n=168 | Neurology n=219 | Rheumatology n=825 | p-value | |
|---|---|---|---|---|---|
| Men, n (%) | 139 (55.6) | 84 (50.0) | 74 (33.8) | 268 (32.5) | <0.001 |
| Age, mean (SD) | 46.0 (16.0) | 43.5 (15.3) | 35.1 (8.9) | 53.9 (15.1) | <0.001 |
| Disease, n (%) | <0.001 | ||||
| Psoriasis | 186 (78.5) | – | – | 2 (0.3) | |
| RA | 2 (0.8) | – | – | 459 (68) | |
| PsA | 49 (20.7) | 1 (0.6) | – | 78 (11.6) | |
| AS | – | – | – | 135 (20) | |
| UC | – | 28 (17.4) | – | 1 (0.1) | |
| CD | – | 132 (82.0) | – | – | |
| MS | – | – | 212 (100) | – | |
Abbreviations: SD=standard deviation; RA=rheumatoid arthritis; PsA=psoriatic arthritis; AS=ankylosing spondylitis; UC=ulcerative colitis; CD=Crohn's disease; MS=multiple sclerosis.
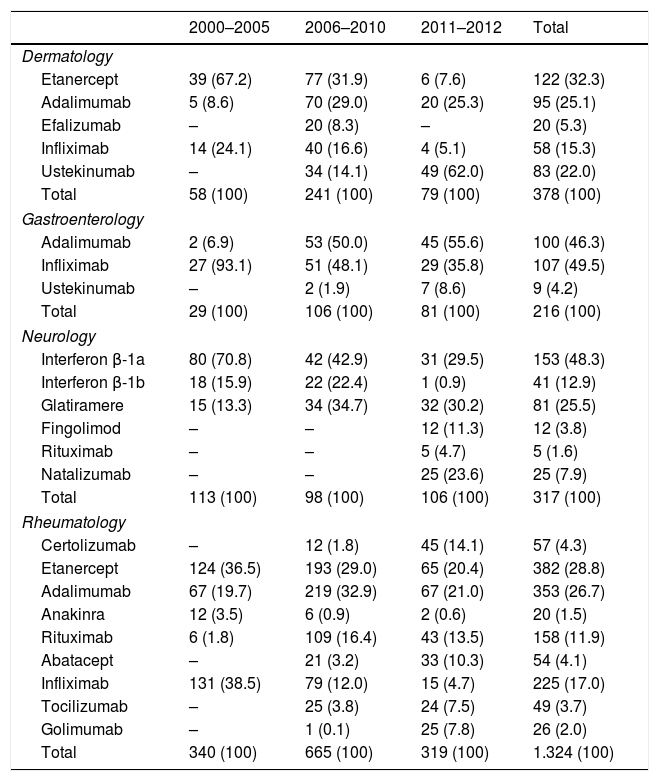
By specialty, the number of treatments analyzed was: dermatology n=378 (16.9%), gastroenterology n=216 (9.7%), neurology n=317 (14.2%), and rheumatology n=1324 (59.2%). In relation to the number of biological treatments per patient, 65% of the treatments analyzed correspond to a first-line treatment, and 22% to a second-line biological; a patient received a maximum of 8 different biologic treatment lines. Table 2 shows the distribution of biological drugs for all lines of treatment by specialty and time-period. The biological agents most frequently used were ETN, ADA, IFX, IFN β-1a, and RTX, and varied within specialty across time-periods.
Biological treatments (all treatment lines), by specialty and period.
| 2000–2005 | 2006–2010 | 2011–2012 | Total | |
|---|---|---|---|---|
| Dermatology | ||||
| Etanercept | 39 (67.2) | 77 (31.9) | 6 (7.6) | 122 (32.3) |
| Adalimumab | 5 (8.6) | 70 (29.0) | 20 (25.3) | 95 (25.1) |
| Efalizumab | – | 20 (8.3) | – | 20 (5.3) |
| Infliximab | 14 (24.1) | 40 (16.6) | 4 (5.1) | 58 (15.3) |
| Ustekinumab | – | 34 (14.1) | 49 (62.0) | 83 (22.0) |
| Total | 58 (100) | 241 (100) | 79 (100) | 378 (100) |
| Gastroenterology | ||||
| Adalimumab | 2 (6.9) | 53 (50.0) | 45 (55.6) | 100 (46.3) |
| Infliximab | 27 (93.1) | 51 (48.1) | 29 (35.8) | 107 (49.5) |
| Ustekinumab | – | 2 (1.9) | 7 (8.6) | 9 (4.2) |
| Total | 29 (100) | 106 (100) | 81 (100) | 216 (100) |
| Neurology | ||||
| Interferon β-1a | 80 (70.8) | 42 (42.9) | 31 (29.5) | 153 (48.3) |
| Interferon β-1b | 18 (15.9) | 22 (22.4) | 1 (0.9) | 41 (12.9) |
| Glatiramere | 15 (13.3) | 34 (34.7) | 32 (30.2) | 81 (25.5) |
| Fingolimod | – | – | 12 (11.3) | 12 (3.8) |
| Rituximab | – | – | 5 (4.7) | 5 (1.6) |
| Natalizumab | – | – | 25 (23.6) | 25 (7.9) |
| Total | 113 (100) | 98 (100) | 106 (100) | 317 (100) |
| Rheumatology | ||||
| Certolizumab | – | 12 (1.8) | 45 (14.1) | 57 (4.3) |
| Etanercept | 124 (36.5) | 193 (29.0) | 65 (20.4) | 382 (28.8) |
| Adalimumab | 67 (19.7) | 219 (32.9) | 67 (21.0) | 353 (26.7) |
| Anakinra | 12 (3.5) | 6 (0.9) | 2 (0.6) | 20 (1.5) |
| Rituximab | 6 (1.8) | 109 (16.4) | 43 (13.5) | 158 (11.9) |
| Abatacept | – | 21 (3.2) | 33 (10.3) | 54 (4.1) |
| Infliximab | 131 (38.5) | 79 (12.0) | 15 (4.7) | 225 (17.0) |
| Tocilizumab | – | 25 (3.8) | 24 (7.5) | 49 (3.7) |
| Golimumab | – | 1 (0.1) | 25 (7.8) | 26 (2.0) |
| Total | 340 (100) | 665 (100) | 319 (100) | 1.324 (100) |
Results are expressed as number and percentage (%).
The agents were used off-label in 271 cases (12.1%). Rheumatology was the specialty with more off-label uses (16.6%) and neurology showed the lowest rate (2.2%). The compounds with the highest percentage of off-label use were ANK (55%), GOL and RTX (both 31%), UST (21%), and IFX (19%), while the proportion did not reach 10% in ETN, ADA, EFZ, or GLA, and it was null for IFN, FGL, and NTZ. Finally, the period with the highest off-label use was 2011–2012 (16%) compared to 2000–2005 (12%).
Drug survivalThe 2238 treatments corresponded to 5309 treatment-years, with a maximum follow-up of 13 years; 1086 treatments were discontinued. The retention rate of all biological treatments was 41% at 5 years, 23% at 10 years and 19% at 12 years.
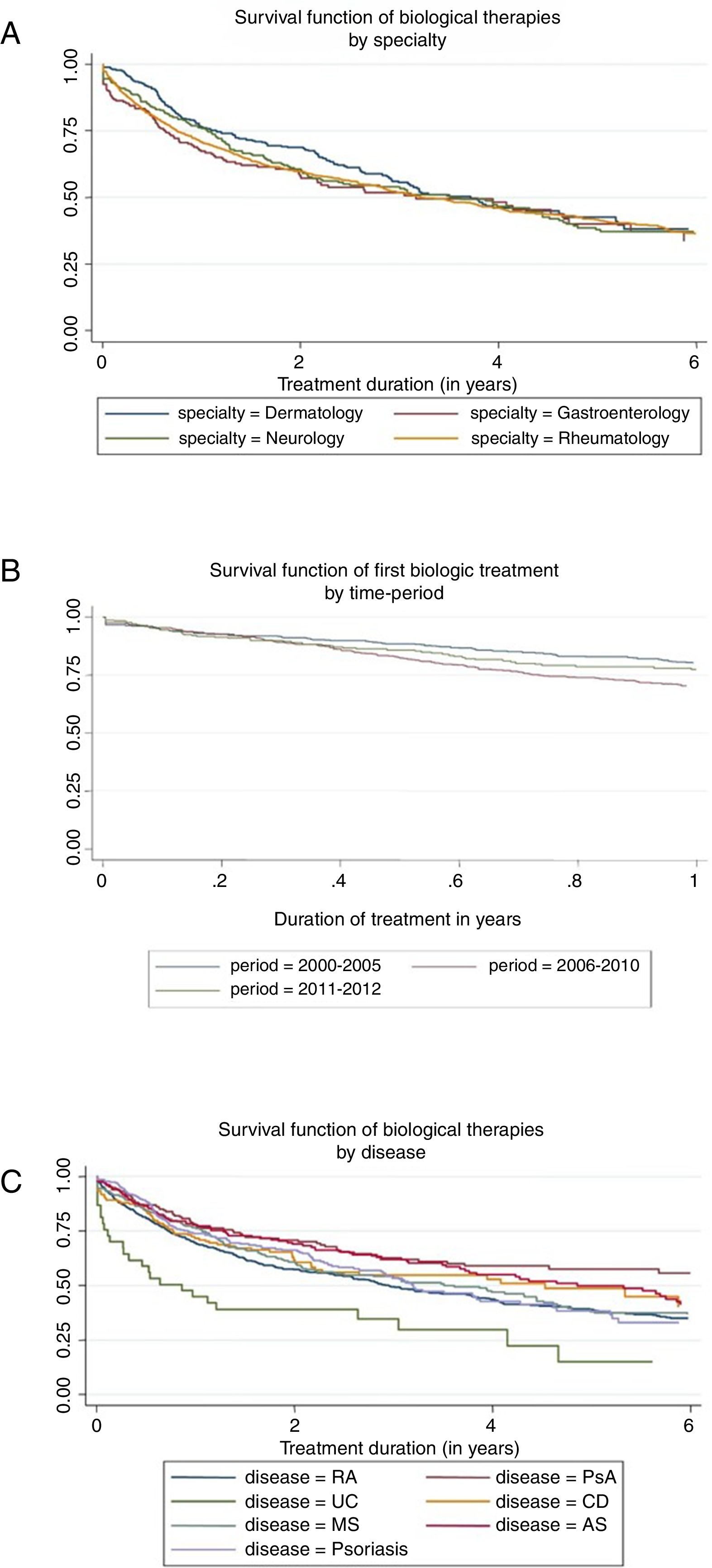
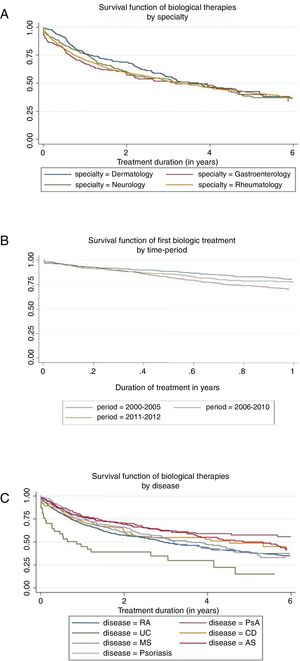
Prior to any adjustment, the highest median survival was observed in dermatology (3.7 years) and the lowest in rheumatology (3.2 years) (see Fig. 1A). At 10 years of treatment, the retention rate was 29% in gastroenterology, 22% in neurology, 17% in rheumatology, and 0% in dermatology, but there were no significant differences between specialties in overall survival (p=0.370).
The median survival differed depending on the underlying disease (p<0.001), the highest being for PsA (7.0 years) and AS (5.3 years), and the lowest for RA (3.0 years) and UC (0.9 years); the median drug survival for psoriasis, MS, and Crohn's disease were 3.2, 3.6, and 4.5 years, respectively (Fig. 1B). After 2 years of treatment start, survival was, in order of frequency, 71% for PsA, 69 for AS, 66% for psoriasis, 62% for CD, 61% for MS, 57% for RA, and 39% for UC.
The analysis of the retention time of the biological compound according to the number of treatment cycles was carried out for the first cycle versus the second and subsequent cycles. The median survival rate goes from 3.85 years for the first cycle to 2.75 years for the second and subsequent cycles (p<0.001). Finally, survival seemed to differ in relation to the time period, but not statistically (p=0.071). Retention rate reached 50% at 4.23 years in 2000–2005 and 3.16 years in 2006–2010—the last period did not reach the median. The retention rate at 1 year was 78%, 67%, and 75% for the period 2000–2005, 2006–2010, and 2011–2012, respectively (Fig. 1C).
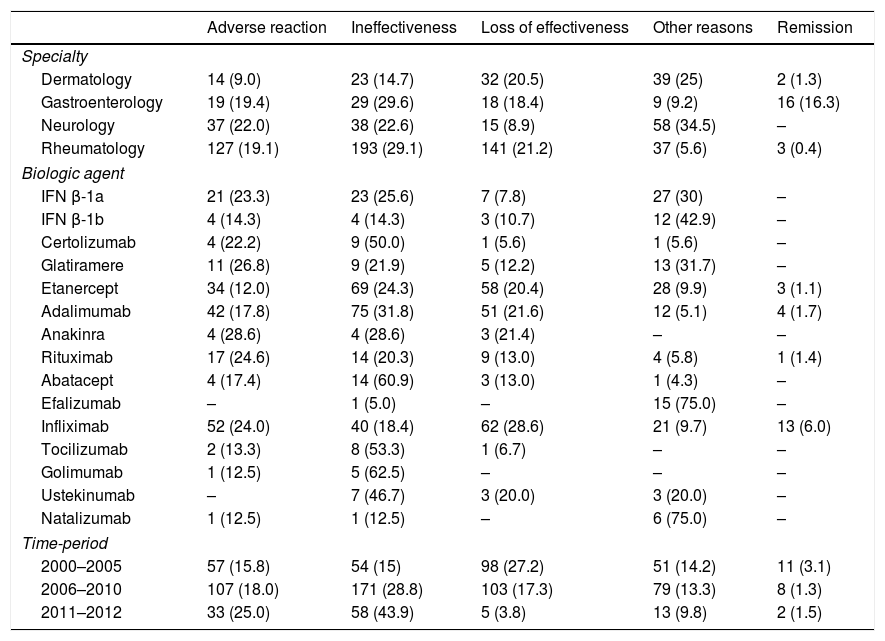
Reasons for discontinuationThe most common reason for discontinuation was ineffectiveness (26%), followed by loss of effectiveness (19%), and adverse reactions (18%). The reasons for discontinuation were significantly different by specialty (Table 3). In dermatology, the most common reason was loss of effectiveness (20.5%), while in gastroenterology and rheumatology this was ineffectiveness (29.6% and 29.1%, respectively), and in neurology, adverse reactions and ineffectiveness showed similar percentages (22% and 22.6%). In relation to drugs, adverse reactions were the most common cause of discontinuation for RTX (24.6%) and IFX (24%), ineffectiveness for ADA (31.8%) and IFN β-1a (25.6%), and loss of effectiveness for IFX (28.6%). Finally, loss of effectiveness was the most common reason for discontinuation in 2000–2005 (27%), while ineffectiveness was the most frequent reason in the other two periods (Table 3).
Reasons for biological treatment discontinuation.
| Adverse reaction | Ineffectiveness | Loss of effectiveness | Other reasons | Remission | |
|---|---|---|---|---|---|
| Specialty | |||||
| Dermatology | 14 (9.0) | 23 (14.7) | 32 (20.5) | 39 (25) | 2 (1.3) |
| Gastroenterology | 19 (19.4) | 29 (29.6) | 18 (18.4) | 9 (9.2) | 16 (16.3) |
| Neurology | 37 (22.0) | 38 (22.6) | 15 (8.9) | 58 (34.5) | – |
| Rheumatology | 127 (19.1) | 193 (29.1) | 141 (21.2) | 37 (5.6) | 3 (0.4) |
| Biologic agent | |||||
| IFN β-1a | 21 (23.3) | 23 (25.6) | 7 (7.8) | 27 (30) | – |
| IFN β-1b | 4 (14.3) | 4 (14.3) | 3 (10.7) | 12 (42.9) | – |
| Certolizumab | 4 (22.2) | 9 (50.0) | 1 (5.6) | 1 (5.6) | – |
| Glatiramere | 11 (26.8) | 9 (21.9) | 5 (12.2) | 13 (31.7) | – |
| Etanercept | 34 (12.0) | 69 (24.3) | 58 (20.4) | 28 (9.9) | 3 (1.1) |
| Adalimumab | 42 (17.8) | 75 (31.8) | 51 (21.6) | 12 (5.1) | 4 (1.7) |
| Anakinra | 4 (28.6) | 4 (28.6) | 3 (21.4) | – | – |
| Rituximab | 17 (24.6) | 14 (20.3) | 9 (13.0) | 4 (5.8) | 1 (1.4) |
| Abatacept | 4 (17.4) | 14 (60.9) | 3 (13.0) | 1 (4.3) | – |
| Efalizumab | – | 1 (5.0) | – | 15 (75.0) | – |
| Infliximab | 52 (24.0) | 40 (18.4) | 62 (28.6) | 21 (9.7) | 13 (6.0) |
| Tocilizumab | 2 (13.3) | 8 (53.3) | 1 (6.7) | – | – |
| Golimumab | 1 (12.5) | 5 (62.5) | – | – | – |
| Ustekinumab | – | 7 (46.7) | 3 (20.0) | 3 (20.0) | – |
| Natalizumab | 1 (12.5) | 1 (12.5) | – | 6 (75.0) | – |
| Time-period | |||||
| 2000–2005 | 57 (15.8) | 54 (15) | 98 (27.2) | 51 (14.2) | 11 (3.1) |
| 2006–2010 | 107 (18.0) | 171 (28.8) | 103 (17.3) | 79 (13.3) | 8 (1.3) |
| 2011–2012 | 33 (25.0) | 58 (43.9) | 5 (3.8) | 13 (9.8) | 2 (1.5) |
Results are expressed as number and percentage (%).
A table in supplementary material shows the results for the three models for discontinuation. The probability of discontinuation by any cause increased significantly with ANK (HR=1.85), UC (HR=2.15) and second-line treatment (HR=1.34), and decreased with UST (HR=0.40) and in PsA (HR=0.64) and AS (HR=0.77). As for the discontinuation for ineffectiveness or loss of effectiveness, the probability was lower in PsA (HR=0.53), AS (HR=0.56), and psoriasis (HR=0.61), and with RTX (HR=0.49), while the risk increased significantly with second-line treatment (HR=1.72). Finally, the probability of discontinuation due to adverse reaction was associated with the use of ANK (HR=2.96) and it was significantly lower in PsA than in RA. The treatment line does not seem to have an effect on the discontinuation rate due to adverse reactions.
DiscussionThe utilization of common therapies by different medical specialties with varying degrees of expertise in their use makes sharing experiences highly recommended. The common goal should be to bring about a more efficient and safe use of these drugs.
In recent years, biological agents have been added to the armamentarium of many chronic inflammatory diseases in various medical specialties.1–4 Biologics registers have provided information of great clinical relevance, especially related to the safety of these drugs.17 However, this new scenario implies an increasing complexity in monitoring and prescribing patterns, as well as in testing the effectiveness and safety of drugs. In addition resource limitations stress the need to use these therapies under cost efficiency criteria.
In this context, the BTU of the Hospital La Princesa was launched with the purpose of contributing to the better use of BT. This multidisciplinary unit has been possible after close collaboration between professionals, underscoring the importance of communication, and it bases its procedures and protocols on efficiency, safety, and cost-effectiveness criteria. Currently we are not aware of multidisciplinary units of this kind in our country. Our intention is to share our experience with as many professionals as possible to produce better units.
Despite an asymmetrical initial acceptance of the idea, the BTU has proven to be valuable in favouring relationship among groups involved in the use of BT and contributing to quality care of patients. Issues like the need to define and pursue a therapeutic objective, the possibility of optimization, the need to look for more cost-effective options to intensifications, the reduction of costs or the encouragement for multidisciplinary approaches to patients are among the benefits observed. Among the limitations, we can mention the need for external financial support.
In a first step, we analyzed the use patterns of the different agents at our centre. What was anticipated as a mere descriptive study prompted discussions. The use of these therapies differs between specialties; the same drugs are used intermittently in some diseases, in a continuous fashion in others and at different dosages. However, drug survival is very similar across specialties, despite treating differently expressed diseases, and using different outcome measures. Two contrary explanations serve to this observed phenomenon: (1) it could be that we are showing just means and actually diseases behave differently, or (2) this group of diseases (immune-mediated inflammatory diseases) shows lower inter-variability than previously thought.
Drug survival, however, differs across diseases, and this may have to do with the existence of therapeutic alternatives—the fewer alternatives, the longer drug survival—or with monitoring schemes that force switching—this might be the case of RA and the strict monitoring precluded by the treat to target strategies18—or true differences in efficacy by indication, or even true differences in safety. We have shown, for instance, the case of UST, with no discontinuation for adverse events and ANK with nearly three times the risk of discontinuation than any other biologic agent, independently of the use. In addition, these drugs are used differently for different diseases and so a final treatment dose may be reflecting a temporary cessation due to surgery, or pregnancy, or to remission, according to the different guidelines used by the specialists.7,19–22
The Spanish Rheumatology Society and Hospital Pharmacy Society have developed a joint consensus on the optimization of biologics in patients with RA, AS, and PsA.20 In the document, the experts from both societies recommend when and how to taper biologic treatment in patients with these diseases based on systematic reviews. We believe that this type of guidelines are necessary to improve the risk–benefit ratio and efficiency of these treatments, and to reduce unwanted variability. As part of the plan of the BTU, we aspire to convey recommendations based on the profiles of patients who do not reach a therapeutic target by objective measures and yet are maintained on therapy, or even tapered their treatment. Precisely a critical aspect will be to understand why physicians who are experts in a certain disease, may not follow guidelines—or apparently as per objective measures.
We assume that this analysis is just a starting point, and that it has multiple shortcomings, a retrospective design, non-protocolised data collection, wide variability within services, etc. However, we consider it a benchmarking analysis to see how difficult the enterprise will be. We have not yet analyzed the costs, but this will be clearly the next evaluation to address.
As a conclusion, the setting of a BTU with a multidisciplinary approach is an interesting initiative that we hope will contribute to a better use of BT. Analysing the situation in our centre is helping us to recognize the challenges and to dimension the problem of sharing expensive medications across specialties; the ongoing discussions on these and further results should foster the collaboration and the knowledge base in BT.
Ethical approval and data sharingAll study procedures were performed in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent was not required. Data related to this paper are accessible upon request to JMAG.
FundingThe BTU is supported by unrestricted funds from Abbvie, Biogen, Bristol-Myers Squibb, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi-Aventis, and UCB Pharma.
Conflict of interestDr. Gisbert has served as a speaker, a consultant and advisory member for, or has received research funding from MSD, Abbvie, Hospira, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma. Dr. Dauden reports advisory Board member, consultant, grants, research support, participation in clinical trials, honorarium for speaking, research support, with the following pharmaceutical companies: Abbvie/Abbott, Amgen, Centocor Ortho Biotech Inc., Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD, Celgene, and Lilly during the conduct of the study. Dr Meca has served as a speaker, a consultant and advisory member for, or has received research funding from Biogen Idec, Roche, Merck Serono, Novartis, TEVA, Sanofi, Genzyme. Dr. Morell Baladron reports educational activities for Novartis Lilly, Roche, Gilead, Abbvie, Biogen, Ferrer, GSK, and Bifort outside the submitted work; Dr. Garcia-Vicuña has served as a speaker, a consultant and advisory member for, or has received research funding from MSD, Abbvie,Pfizer, UCB, Roche, Hospira, Janssen, Sandoz, during the conduct of the study; InMusc holds research contracts with Abbvie, Roche, MSD, Pfizer, and BMS. Dr. Alvaro-Gracia reports grants and personal fees from Abbvie, grants and personal fees from BMS, grants and personal fees from MSD, grants and personal fees from Pfizer, grants and personal fees from Roche, grants and personal fees from UCB, personal fees from Janssen, grants and personal fees from Novartis, personal fees from Tigenix, during the conduct of the study.