Systemic lupus erythematosus (SLE) and the vasculitides associated with antineutrophil cytoplasmic antibodies (ANCA) are well-differentiated diseases; although approximately a third of the patients with SLE are ANCA-positive,1 this is rarely associated with vasculitis. The presence of ANCA in patients with SLE seems to predispose them to lupus nephritis (LN), low levels of complement C3 and a higher rate of complications.2
We present the case of a 55-year-old man from Mexico City, who presented with a 5-month history of arthralgia in shoulders, wrists and ankles, that was bilateral and symmetrical; moreover, he had episodes of recurrent sinusitis, weight loss and edema arising from the lower limbs; there were no lesions affecting the skin. In the initial examination he underwent urinalysis which showed proteinuria of 200mg/dL, innumerable erythrocytes, leukocytes 20 to 25 per field, red blood cell and leukocyte casts; creatinine: 1.66mg/dL (reference range 0.6–1.3mg/dL). Blood tests revealed hemoglobin concentration of 9.1g/dL (reference range 13–17g/dL), normocytic and normochromic; the remaining cell lines were normal; erythrocyte sedimentation rate (ESR): 55mm/h (reference range 0–15mm/h); C-reactive protein (CRP): 6.24mg/dL (reference range 0–3mg/dL). Immunological profile with a 1:160 titer of antinuclear antibodies (ANA), which had a homogeneous/fine speckled pattern by immunofluorescence and 78.0IU/mL in enzyme-linked immunosorbent assay (ELISA) (reference range for high positive >60IU/mL), anti-double stranded DNA (anti-ds DNA) by chemiluminescence: 1.4IU/mL (reference range for negative <20IU/mL), antiproteinase 3 (cytoplasmic [c-ANCA]) positive with titers reaching 1:320, granular pattern by immunofluorescence and higher than 100IU/mL by ELISA (reference range 0–3.5IU/mL), myeloperoxidase (perinuclear [p-ANCA]) were negative using the same technique, complement C3 was 71.7mg/dL (reference range 90–180mg/dL) and complement C4 was 17.6mg/dL (reference range 10–40mg/dL); human immunodeficiency virus (HIV) antibodies, cryoglobulins and hepatitis B and C negative; subsequently, 24h urine was collected and revealed a proteinuria of 1.77g.
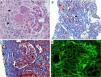
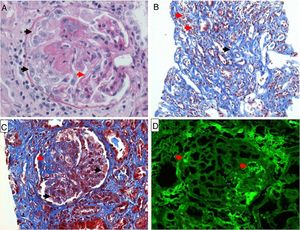
Given the presence of data compatible with nephritic syndrome, the patient underwent renal biopsy. The result was membranoproliferative and active diffuse extracapillary glomerulonephritis (crescents 80%) due to immune complexes, interstitial fibrosis, tubulointerstitial nephritis with mononuclear infiltrate, with no evidence of vasculitis; direct immunofluorescence with deposits of immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin M (IgM), complement C1q, complement C3 with a focal granular pattern (Fig. 1). With these data associated with the clinical presentation, the diagnosis was LN class IV-S (A), with an activity score of 18 and chronicity of 7.
Renal biopsy. (A) Hematoxylin–eosin staining 40×; observe the membranoproliferative pattern with an image showing double contour (red arrow), formation of crescents (black arrows). (B) Masson trichrome staining 10×; observe tubulointerstitial nephritis with mononuclear infiltrate (red arrows) and interstitial fibrosis (black arrow). (C) Masson trichrome staining 40×; with presence of thrombi and fibrinoid necrosis in capillary loops (black arrows), segmental glomerular fibrosis (red arrow). (D) Immunofluorescence 40×; positive for immunoglobulin G with a focal granular pattern and only a few segments of capillary loops (red arrows).
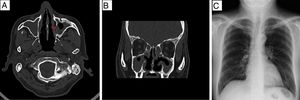
Treatment was begun with 1g intravenous cyclophosphamide every 30 days for a total of 6 doses, 1g intravenous methylprednisolone every 24h the first 3 days, followed by 60mg of oral prednisone every 24h for 4 weeks, 24-hour monitoring of proteinuria 1 month after treatment of 870mg, creatinine of 1.54mg/dL, at which time a trend toward a partial response was established.3 The patient continued with cyclophosphamide and steroids were tapered. During follow-up, there was an increase in proteinuria, persistence of dysmorphic erythrocytes at 0–5 per field, monitoring of positive c-ANCA at a titer of 1:160 in immunofluorescence, aside from a reduction in the glomerular filtration rate, with an increase >1mg/dL in creatinine, leading to renal relapse.3Table 1 summarizes the 6-month follow-up. In line with the diagnosis of LN, given the symptoms of chronic sinusitis and positive c-ANCA, we studied a possible associated vasculitis. We performed computed tomography of the paranasal sinuses 20 days after the initiation of treatment (Fig. 2), which revealed swelling of the nasal mucosa, with slight bone erosion in left maxillary antral wall and turbinates justified by the chronic sinusitis process; chest radiograph was normal (Fig. 2C), and a biopsy of the nasal mucosa showed the absence of granulomas or other data suggesting a process of vasculitis.
Changes in 24-hour Proteinuria, Serum Creatinine, Cytoplasmic Antineutrophil Cytoplasmic Antibodies and Complement Over a 6-month Follow-up Period.
| Diagnosis | First month | Third month | Sixth month | |
|---|---|---|---|---|
| 24-Hour proteinuria (mg) | 1770 | 870 | 4055 | 2967 |
| Serum creatinine (mg/dL) | 1.66 | 1.54 | 2.57 | 2.48 |
| C-ANCA by immunofluorescence | 1:320 | 1:160 | ||
| Complement C3 (90–180) (mg/dL) | 71.7 | 85.6 | 73.2 | 106.0 |
| Complement C4 (10–40) (mg/dL) | 17.6 | 26.8 | 18.4 | 28.2 |
C-ANCA, cytoplasmic antineutrophil cytoplasmic antibodies.
(A and B) Computed tomography images of paranasal sinuses in simple phase: axial scan (A), coronal scan (B), swollen nasal mucosa, slight bone erosion involving left maxillary antral wall (red arrow) and inferior turbinates. (C) Normal posteroanterior chest radiograph; no lesions on lung parenchyma.
This case corresponds to LN with failure of the first line of treatment, associated with high titers of positive c-ANCA, with no evidence of solid elements enabling the parallel diagnosis of vasculitis. A number of authors have observed that SLE patients can present these antibodies; that is the case of Galeazzi et al.,4 who evaluated 566 patients with SLE in 11 European centers, presenting a prevalence of 16.4% (15.4% with p-ANCA and 1% with c-ANCA) in individuals in whom that relationship was detected; other reports demonstrate a highly variable relationship with prevalence of up to 37.3%,5,6 predominantly with p-ANCA positivity.
We recommend that all the patients with LN should undergo an intentional search for ANCA, because they have a higher positivity rate than SLE patients with no renal involvement; Pradhan et al.7 recorded a prevalence of 54.5%, and all of these individuals were positive for p-ANCA; subsequently, Pan et al.2 evaluated 60 patients with a diagnosis of SLE, 28 of them with LN, observing a prevalence of ANCA of 53.6% and concluding that said association was related to higher activity and a poorer prognosis. Nasr et al.8 reported 10 patients with LN associated with the presence of ANCA and described the histopathological features of the renal biopsy. It revealed a greater formation of crescents in our patient, involving 80% of the glomeruli, and areas of necrosis were also demonstrated by the histopathological study (Fig. 1). This suggests that their presence precipitated those findings, although the role they play in the pathophysiology of LN has not been made clear.
With regard to treatment, the population with LN and ANCA-positivity should undergo follow-up studies of the scheme for the induction of conventional remission; given the failure of complete remission with first-line treatment in our patient, an alternative strategy should be proposed for initial treatment for patients of this type.
Please cite this article as: Tobar-Marcillo M, Destruge-Molina I, Torres-Orozco L, Santiago-Ramirez R. Nefritis lúpica asociada con c-ANCA. Reumatol Clin. 2018;14:246–248.