The relationship between the pharmaceutical industry and physicians is evermore complex and has called attention on both sides.
It is evident that, for many, medical ethics, both in dealings and empathy with patients as with the relationship between the pharmaceutical industry and the government, which in their current state are undesirable. Therefore, there is an urgent need for guidelines that define the noblest path in order to reach an outcome that benefits the patient. This might be achieved through adequate attitudes and agreement on the part of physicians, by knowing, adapting, and counting with economic requirements for health expectations, with legislation and recommendations for patient inclusion in international trials, with medical error preventive measures, knowing the role of pharmaceutical companies’, cost of developing drugs and advantages and disadvantages of generics, to name a few of the main needs.
For objective data of the impact and acceptance of relationships between industry and physicians, there have been several polls that show objective geographic and population data, with reasonable consistency that could define potential conflicts of interest. In surveys conducted in the United States of America (USA), as in Japan, continuing medical education with promotional events and meals is accepted as common practice (sponsored by the pharmaceutical industry), even in the workplace (67%–83%), but there is less approval for major gifts (25%).1,2 Ninety-six percent of Japanese physicians accept drug samples and half accept subsidized medical events. Despite this, doctors deny the influence on their personal requirements but accept that this conduct on their part and the pharmaceutical industry do influence their behavior.3 There is acceptance of these figures and an openness on the part of physicians in the influence the pharmaceutical industry has, both consciously and unconsciously, on prescriptions. The health professional should also note that, although it benefits medical excellence and may promote the ideal outcome measures mentioned above, the pharmaceutical industry is also interested in increasing their sales and that the responsibility for the best selection of medicines lies with the medical professional.
In general, we recognize that the actions of the pharmaceutical industry may be not only appropriate but also necessary, which is represented in controlled clinical studies of large numbers of patients and reasonable follow-up time, which would otherwise be impossible to achieve, given the high cost of research accounting, but is very difficult to disassociate it from a potential “conflict of interest” (circumstances that influences professional judgment or actions considered primary interests and may be influenced by secondary interests).4,5
In the United States, the Senate has tried for 50 years to legislate (Kefauver legislation) on the prescription by doctors in order to achieve goals of equity, efficiency, balance, and medical competence regarding ethics.6 This has been regulated trough the European Drug Agency, the FDA, the International Committee of Research, and New Drug Application Medical Journal Editors, the operational validity, vying for internal consistency and certification of data, among other parameters, and leading to the goals of adequateness and not obtaining or interpreting analysis of results that disagree between what is observed and published.7 In fact, despite adequate agreement between patients and physicians, with emphasis on engagement, recommendations, recruitment, selection, support, and knowledge, in the acceptance of trial participation and making the best informed decision, nearly one third of patients opt out of alleged financial conflicts of interest.8,9
There are undeniable efforts, difficulties, challenges, and costs in clinical research studies. However, only 43% of drugs approved by the FDA undergo double-blind, controlled and excellently planned research studies culminating in high impact medical journals, and this occurs more frequently when the findings are positive and show adequate statistical differences. In the case of negative results, it is interesting to note that most are not published or show positive results or trends that do not necessarily relate to the primarily raised objectives. The publications of relevant studies sponsored by industry is variable and range from 7% in British Journal of Medicine to 32% in the New England Journal of Medicine. The pharmaceutical industry participates with more than a third of the cost of continuing medical education in the U.S. (between 9 and 14 billion dollars). Despite this, and even contrasting with the economic effort, we must highlight that the persistence and prevalence of adverse events reported to the Institute of Medicine 7000 to 98000, with an annual cost of 1.5 million USD.10,11
Of the main governmental expenditure items, those related to health, in particular those derived from drugs, are highest, so various governments have been taking steps to limit these stratospheric costs, as in the U.S. Competition among pharmaceutical companies and diversification of the market with the introduction of generic drugs, resulting from the loss of patents, could help reduce these costs. Recently, El Pais, a Spanish newspaper, noted that a monopoly could lead to inappropriate medical practices and, in recent years, limits in prescriptions of original brand drugs led to freer prescription and potential savings associated with prescription of generics, which could limit the number of pharma representatives.12 The influence of pharmaceutical companies can lead to conflicts of interest, but in the U.S. probably the worst influence is the fear of the lawsuit; patients requiring opioids, for example, have increased and also resulted in a substantial increase in the number of hospitalizations.13,14 In fact, misuse or abuse of prescription drugs are responsible for a substantial increase in overdose deaths (75%) >1.2 million/year and emergencies.15
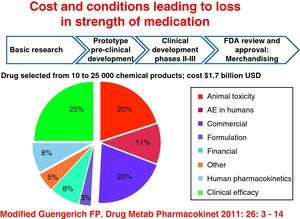
All of this is regardless of the cost resulting from research for selection of a drug from between 10000 and 25000 chemical compounds, which translates into 1.7 billion dollars and is greater with biological therapy in rheumatology, and in other branches of medicine, this includes the cost of the drug since its inception, experimental animal studies and subsequently phases I, II, and III, to FDA approval and release. Also of interest are the determinants of loss of strength of medicines derived from animal and human toxicity, resulting in adverse events and loss of clinical efficacy, which hardly goes beyond 25% (Fig. 1).16
Even without defining actions and appropriate responses to the above mentioned, we have questions regarding generic drugs that have no satisfactory answers at all, so in terms of efficacy, which is lower by 10%–20% compared to the original, their transcendence can be potentially different between the different medications, so it is not the same to talk of anti-inflammatory drugs than anticonvulsants or bisphosphonates, as outcome measures are different so, for the first, the objective is the response in terms of pain and swelling, for antiepilepsy, the lack of crises and their consequences, and for the latter, the rate of fracture and its complications in terms of disability or death. This leads to the acceptance that the differences go beyond just considering plasma levels, pharmacokinetics, pharmacodynamics, and costs between generics and originals. Regarding biological products, today our level of ignorance is even greater in the absence of long term, controlled trials for generics.17,18
We consider that physician attitudes and acceptance of pressure from the pharmaceutical industry must be limited or regulated, that expectations on health expenditure are greater that what is currently derived for such an end, that legislation must be drawn up derived from patient and physician needs, that patient inclusion in international trials must be the main outcome in benefit of the patient and that we have an obligation to increase continuing medical education in order to prevent negligence and, additionally, include these warning from pregraduate studies. With these actions we could avoid or substantially limit negligence and lawsuits that modify good clinical practice and elevate demand of additional studies and, therefore, costs, taken as a defensive posture.19,20
Please cite this article as: Abud-Mendoza C. Ética médica, investigación y la industria farmacéutica. Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2011.12.010.