There is a wide variability in the diagnostic and therapeutic methods in rheumatoid arthritis (AR) in Spain, according to prior studies. The quality of care could benefit from the application of appropriate clinical practice standards; we present a study on the variability of clinical practice.
MethodsDescriptive review of clinical records (CR) of patients aged 16 or older diagnosed with RA, selected by stratified sampling of the Autonomous Communities in two stages per Hospital Center and patient. Collected analysis of sociodemographic data, evolution, follow-up, joint count, reactants, function, job history, Visual Analogue Scales (VAS) and other.
ResultsWe obtained valid information of 1272 RA patients. The ESR, CRP and rheumatoid factor (RF) were the regularly used parameters. The percentages of missing data in tender (TJN) and swollen (SJN) joint counts were 8.2% and 9.6%, respectively; regarding the VAS we found 53.6% (patient), 59.1% (pain), and 72% in the physician VAS.
ConclusionsDespite having clinical practice guidelines on RA, there still exists a significant variability in RA management in our country.
Los resultados de estudios previos muestran una amplia variabilidad en los medios diagnósticos y terapéuticos en artritis reumatoide (AR) en España. La calidad asistencial se beneficiaría al aplicar estándares de práctica apropiados; se presenta un estudio sobre variabilidad en el manejo de la AR en España.
MétodosEstudio descriptivo de revisión de historias clínicas (HC) de pacientes con AR de edad mayor de 16 años, seleccionados por muestreo estratificado por comunidades autónomas y bietápico por centro hospitalario y paciente. Se recogió datos sociodemográficos, evolución, seguimiento, recuento articular, reactantes, función, vida laboral, escalas visuales analógicas (EVA) y otros.
ResultadosSe obtuvo información válida de 1.272 pacientes con AR. Se empleó mayoritariamente la VSG, PCR y factor reumatoide (FR). Los porcentajes de ausencia de datos en los recuentos de articulaciones dolorosas (NAD) y tumefactas (NAT) son el 8,2 y el 9,6%; se utilizaron poco las EVA.
ConclusionesA pesar de tener una guía de práctica clínica sobre la AR, existe variabilidad en su manejo.
Variations in clinical practice (VCP) are defined as differences in the care process and/or outcome of care of a particular clinical problem among different providers, after controlling for demographic, sociocultural and health1 status. The study of the problem of variability in medical practice originated with the work of Wennberg and Gittelshon.2,3 It is recognized that VPC is influenced by several factors, including the inaccuracy of the data or its treatment,4 those related to demand of care,5 characteristics of health professionals6 and the health system itself.7 We also know that comorbidity and disease characteristics influence the clinical expression, but we need to know whether these effects are due to modifiable factors.8 All these data justify VCP evaluation studies for a particular disease.8–10 In this sense, the results of the first study of variability in the treatment of RA in Spain (eMAR I), made 10 years ago, showed wide variation in the use of different health resources, diagnostic and therapeutic procedures and ways of monitoring RA patients which, in many cases, were independent of the characteristics of the patient or the severity of the disease.11,12 On the other hand, due to possible genetic or environmental factors variations have been described in the prevalence of RA, as well as in its clinical expression in different populations with the same geographical origin geográfico.8 To explain this VCP there are 3 theories: demand of attention is attributed more importance (the prevalence of the process in a given area, the population age, socioeconomic status),13 or affects demand of services,14 professional uncertainty exists (lack of scientific evidence on the procedures) physicians favor a certain procedure.15 VCP is common in medicine and causes improper use of procedures, with negative impact on resource consumption and possible adverse effects for patients. The objective of this paper is to describe the clinical characteristics, activity, work disability and comorbidity of RA patients in Spain. Data are from the eMAR II, a study of variability in the treatment of RA and spondyloarthritis (SPA), as measured by various indicators, and dependent on individual factors, disease and health care.
MethodsThe eMAR II is a cross-sectional association study that cross-references variability in the management of RA and SPA and various factors.16
Selection of the Study PopulationThe sample consisted of patients with RA or SPA treated in Rheumatology Spanish hospitals or services with at least one visit to a rheumatologist in the 2 years preceding the date of study onset. Stratified sampling was carried out by autonomous region (cc. AA). A two-stage approach by hospital (first-level unit [UPE]) and patient (second level unit [USE]) was employed. To avoid the lack of representation associated with the homogeneity of UPE, the first stage involved sampling with probability proportional to its size and the second carried out a random, equiprobabilistic selection of patients in each center. The sample size was calculated according to the hypothesis that the proportion of patients who have needed surgery has risen from 18% in eMAR I to 26% in eMAR II. Under this assumption and assuming an alpha error of 5%, a power of 80%, 15% of localized or incomplete stories, with a design effect of 2.5, we obtained a sample size of 1410 patients for each of the study arms. In this study we only consider the RA study arm. Information on general data was obtained from the patient history: date of birth, sex, date of onset of first symptoms, the first visit to a rheumatologist and diagnostic performance of the ACR criteria, ACR functional class, positive factor Rheumatoid arthritis (RF), and cyclic citrullinated peptide (CCP), radiological progression and extra-articular manifestations. The specific progression was assessed by different parameters: acute phase reactants (maximum and minimum values of ESR, CRP), visual analog scales (VAS) with the best and worse subjective physician and patient assessments of disease activity (no VAS activity was <10mm or complete remission, either medically or through some objective criterion; mild when the EVA was ≥10 and <40 or patients had mild activity that did not require treatment modification; moderate when the VAS was between ≥40 and <60 or patients had required minor modification of the treatment, such as transient increase doses of NSAIDs or corticosteroids; severe when the VAS was ≥60 or major modification of treatment, such as increased dose, addition or change of a DMARDS was needed), minimum and maximum values of the pain and activity VAS, and the number of tender (TJC) and swollen (SJC) joints, minimum and maximum duration of morning stiffness, minimum and maximum of Disease Activity Score (DAS-28) and Health Assessment Questionnaire (HAQ) score. In addition, information was collected on frequency of use of different procedures for clinical follow-up (with options: the patient never underwent a procedure, occasionally if it was less than 25%, usually if it was between 25% and 75%, and always if it was over 75% of visits), joint counts (28 joints or other), pain assessment by the physician and the patient (VAS or other procedures), acute phase reactants (ESR, CRP or other), compound activity scores (DAS, SDAI or others), functional capacity (functional class ACR,17 HAQ), comorbidity, active working status over 50% in the past 2 years, patient characteristics (education level, occupation, residence) and the physician responsible. Although not employed in this work, the data collection sheets (HRD) also collected extensive information on resource consumption, treatment with NSAIDs, analgesics, corticosteroids, slow acting antirheumatic drugs (DMARD), injections and other medications, biological drugs as well as gastric and osteoporosis prophylaxis.
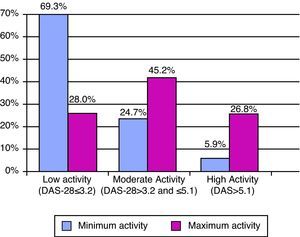
Statistical AnalysisA descriptive study was conducted using central tendency (mean or median) and dispersion measures (standard deviation and 25 and 75 percentiles) for continuous variables, adjusted or not according to the distribution, and percentages for qualitative variables. We classified DAS-28 into 3 activity levels: low (DAS≤3.2), moderate (3.2–5.1 DAS) and high (DAS>5.1). The statistical program employed was Stata 9.0 (StataCorp, College Station, USA).
ResultsSociodemographicFrom a theoretical sample extract (No.=1410) we obtained valid information from 1272 patients with RA, representing 90.2% of the sample. In Table 1, presents the sociodemographic characteristics.
Sociodemographic Characteristics of Patients With RA.
| Characteristic | Median (p25–p75) or No. % | Not Present in Patient History, No. (%) |
| Current age, years | 63.3 (51.6–73.3) | |
| Age at onset of disease | 49.8 (23.2–39.8) | |
| Time since onset, months | 94.8 (46.2–167.9) | |
| Gender (No.=1267) | ||
| Male | 339 (26.8) | |
| Female | 928 (73.2) | |
| Marital status (No.=1263) | 733 (58.0) | |
| Single | 49 (3.9) | |
| Married | 397 (31.4) | |
| Widower | 67 (5.3) | |
| Separated | 17 (1.3) | |
| Level of schooling (No.=1257) | 903 (71.8) | |
| None | 30 (2.4) | |
| Primary | 217 (17.3) | |
| Secondary | 58 (4.6) | |
| Superior | 49 (3.9) | |
| Profession (No.=1251) | 657 (52.5) | |
| Administration | 5 (0.4) | |
| Technical, professional, intellectual | 24 (1.9) | |
| Technical and professional support | 22 (1.7) | |
| Services | 44 (3.5) | |
| Agriculture and fishing | 41 (3.3) | |
| Industry | 13 (1.0) | |
| Operators and assemblers | 42 (3.3) | |
| Unqualified workers | 20 (1.6) | |
| Armed forces | 101 (8.1) | |
| Homemakers | 14 (1.1) | |
| Students | 262 (20.9) | |
| Residency (No.=1266) | 56 (4.4) | |
| Same locality | 666 (52.6) | |
| Different locality | 544 (43.0) | |
| Distance to hospital (N=542) | ||
| Less than 20km | 188 (34.7) | |
| Between 20 and 50km | 204 (37.6) | |
| Over 50km | 130 (24.0) | |
| Unknown | 20 (3.7) | |
| Active working status>50% (No.=1161) | 632 (54.4) | |
| Yes | 401 (34.5) | |
| No | 128 (11.0) | |
| Disability periods (No.=460) | 393 (85.4) | |
| Yes | 33 (7.2) | |
| No | 34 (7.3) | |
| Number of disability episodes | 2 (1–2) | |
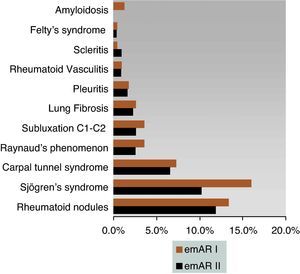
93.4% of patients met ACR 1987 criteria for classification. Most patients were in functional class I (36%), while significantly lower proportions were located in functional classes II (16.3%) and III (11.3%). The limitation for any type of activity only occurred in 6.4% of patients and no data on functional class were found in 29.9%. RF was positive in 73.9% of cases, while only 41.3% had positive CCP. These differences were maintained owing to the lack of consistency of these in the patient history (1.3% for RF compared with 40.6% for CCP). The use of RF had a median (p25–p75) 3 (1–5), while CCP values were 0 (0–1). A significant proportion of the cases had erosive disease (58.7%), and no radiological study was performed in 4.8%. Extra-articular manifestations were found in 306 (24.1%) patients, 2 in 71 (5.6%), 3 in 16 (1.3%) and 4 in 4 (0.3%) patients, and 31, 3% had no extra-articular manifestations (Fig. 1).
Evaluation of Activity and Functional CapacityThe acute phase reactants employed in a methodical way were ESR (77.8%) and CRP (75.1%), the median values varying (p25–p75) from minimum 11 (5–20) to maximum 33 (18–51) in the case of ESR, and from 0.3 (0.2–0.9) to 1.5 (0.2–0.9) for CRP. The assessment, by VAS pain and activity by the patient, showed similar values between the two scales, with minimum values according to the patient of 20 (6–30) for pain and 17 (5–30) for activity, and a maximum of 50 (25–70) for pain and 50 (27–70) for activity. When a physician performed the evaluation of the activity, the minimum and the maximum were 10 (5–20) and 40 (14–60), respectively. The best and worst assessments of the activity of the patient showed distribution patterns similar to those made by their doctors. Morning stiffness minimum was 0 (0–10) and maximum of 20 (0–60)min. The percentages of patient histories with no data in the assessments mentioned above were 59.1% for the patient activity VAS, 72% for activity VAS according to the physician and 50.5% for morning stiffness.
The minimum and maximum values of the DAS-28 showed low activity with a median score (p25–p75) of 2.5 (1.9–3.5) and moderate score of 4.1 (p25–p75) (3.0–5.2) (Fig. 2). In parallel, functional capacity according to HAQ was well preserved, with minimum and maximum values of 0.4 (0–1.0) and 1.0 (0.4–1.6), respectively. There were no HAQ data in 86.6% of the medical reviews. The DAS data were not found in the history of the patient in 55% of the sample in the case of patients treated with biologics, and had no DAS before the start of the treatment in 47.3% (222 of the 469 cases who initiated biologic) of those who received it. Despite the high number of missing values in both measurements, there was only a simultaneous absence of both in 207 cases, representing 44.1% of patients receiving biologics.
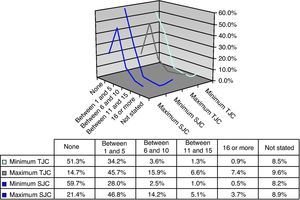
Joint CountsThe majority of patients had a minimum TJC of 0 (51.3%) or between 1 and 5 (34.2%) and a maximum number of 1–5 (45.7%) or between 6 and 10 (15.9%). The distribution of the minimum SJC was symmetrical to that of TJC, with the majority of patients having no affected joints or 1–5. Similarly, it symmetry was observed between the maximum count of TJC and SJC, with most of the patients in the category of between 1 and 5 joints, and with the minimum values between 11 and 15, or over 16 (Fig. 3).
Clinical Follow-upRegarding the various clinical monitoring procedures used, results showed that the physicians assessed joints by counting 28 always (41.4%) or never (57.2%). On the contrary, it is noteworthy that VAS in general was not used to assess pain, by the physician or by the patient, as shown in Table 2, although acute phase reactant testing was performed (ESR and CRP). The responsiveness of the best subjective assessment of disease activity by the physician and the worst subjective assessment of the activity are shown in Table 3.
Degree of use for Clinical Follow up Instruments (Frequency: No., [%]).
| Procedures, Other Procedures, Evaluations and Reactants | Never | Occasionally | Commonly | Always |
| 28 joint count (No.=1231) | 172 (14.0) | 231 (18.8) | 318 (25.8) | 510 (41.4) |
| Joint count: other (No.=787) | 450 (57.2) | 102 (13.0) | 116 (14.7) | 119 (15.1) |
| Evaluation of pain by the physician: VAS (No.=1217) | 718 (59.0) | 217 (17.8) | 248 (12.2) | 134 (11.0) |
| Evaluation of pain by the physician: other (No.=821) | 518 (63.1) | 95 (11.6) | 133 (16.2) | 75 (9.1) |
| Evaluation of pain by the patient: VAS (No.=1225) | 540 (44.1) | 257 (21.0) | 219 (17.9) | 209 (17.1) |
| Evaluation of pain by the patient: other (No.=808) | 487 (60.3) | 79 (9.8) | 134 (16.6) | 108 (13.4) |
| Acute phase reactant: ESR (No.=1259) | 17 (1.3) | 62 (4.9) | 201 (16.0) | 979 (77.8) |
| Acute phase reactant: CRP (No.=1219) | 38 (3.1) | 97 (8.0) | 169 (13.9) | 915 (75.1) |
| Acute phase reactant: other (No.=676) | 410 (60.6) | 108 (16.0) | 47 (6.9) | 111 (16.4) |
| Compound score: DAS-28 (No.=1226) | 592 (48.3) | 226 (18.4) | 226 (18.4) | 182 (14.8) |
| Compound score: SDAI (No.=1053) | 1013 (96.2) | 14 (1.3) | 18 (1.7) | 8 (0.8) |
| Compound score: other (No.=833) | 763 (91.6) | 26 (3.1) | 35 (4.2) | 9 (1.1) |
| Functional capacity: HAQ (No.=1218) | 747 (61.3) | 222 (18.2) | 150 (12.3) | 99 (8.1) |
| Functional capacity: other (No.=888) | 704 (79.3) | 52 (5.9) | 83 (9.3) | 49 (5.5) |
Comparison in Use of Clinical Follow-up Instruments on Studies Regarding Variability in Management of RA, No (%).
| Study | Evaluation of Patient Activity,a No. (%) | Evaluation of Physician Activity,a No. (%) | HAQb | TJC | SJC | 28 Joint Countb | Morning Stiffness |
| emAR II | 328–65 (26–5.1) | 350–75 (27.9–5.9) | 61.3 | (Fig. 3) | (Fig. 3) | 14.0 | N.C. |
| 421–238 (33.3–18.9) | 413–251 (33–19.9) | 18.2 | 18.8 | 50.5% | |||
| 164–335 (13–26.6) | 121–341 (9.6–27.1) | 12.3 | 25.8 | ||||
| 27–289 (2.1–22.9) | 14–222 (1.1–17.6) | 8.1 | N.C. | N.C. | 41.4 | ||
| 321–333 (25.4–26.4) | 355–371 (28.3–29.4) | 9.0 | 8.0 | ||||
| Bellamy et al.19 | |||||||
| Never | 29 | 21 | 16% | 5 | 3 | 10 | 2 |
| Occasional | 20 | 13 | 13 | 13 | 15 | 5 | |
| Commonly | 26 | 34 | 40 | 38 | 37 | 31 | |
| Always | 25 | 32 | 42 | 46 | 38 | 62 | |
| emAR I29 | |||||||
| Never | 707 (51.3) | 536 (38.8) | 76.9 | 4.0 | 2.4 | 85.5 | 15.9 |
| Occasional | 246 (17.8) | 248 (17.9) | 12.7 | 8.4 | 8.5 | 7.3 | 17.6 |
| Commonly | 227 (16.4) | 357 (25.9) | 8.6 | 36.2 | 37.9 | 2.8 | 30.5 |
| Always | 199 (14.3) | 238 (17.1) | 1.5 | 51.2 | 51.0 | 1.1 | 35.8 |
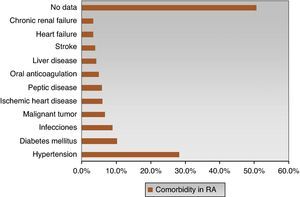
The most frequent comorbidities were hypertension (28.2%) and diabetes (10.2%). However, there was no evidence of comorbidities in 50.6% of cases of RA (Fig. 4), which does not mean that patients did not have other comorbid situations not covered by the collection instrument. In eMAR I, 37% of patients had some comorbidity,18 hypertension being the most frequent (20%), followed by peptic disease (14%), diabetes (7%) and, less frequently, renal failure, liver disease and anticoagulant therapy complications (3%).
Impact on Work Disability34.5% of patients with RA had an active working life for more than 50% of the study period, with data on temporary disability periods shown in Table 1.
DiscussionRegarding the clinical features of RA, in a comparison between the results obtained in the study eMAR II and the eMAR I, no great changes are seen in some parameters, such as age (mean±SD) with values 62.0±14.5 years vs 61.3±13.5 years; percentage of women 73.5% vs 73.2%, RF positivity 74.0 vs 75.9%, and average time since onset in months 122.8±107.3 vs 127.6±97.6, respectively.18 However, statistically significant differences regarding functional status as measured by functional class was seen in eMAR II patients in relation to eMAR I, with a significantly better functional status, more often than in class I (36% vs 27%) and with the lowest proportion in class II (16.3% vs 40%) and III (11.3% vs 26%), although in class IV the results are similar (6.4% vs 6.5%).18 In addition, as constantly stressed, the absence of information in the history of a significant proportion of patients was seen in both studies (31.2% vs 29.9%). The scarcity of data in the histories reviewed is similar to that found when comparing this study to Bellamy et al., as the functional class is used only in 49% of the sample, followed by HAQ in 16%.19 The absence of DAS data could be evaluated as part of a variability justified by the features of the disease, and milder patients may not require collecting these data because of a lack of indications for biological treatment. However, in cases where biological treatment was initiated, the absence of DAS before the introduction of this treatment occurred in 53% of patients, and can be seen as an unjustified variability.
Regarding laboratory tests, the study by Donald et al. shows that, in RA, these parameters are used by 86% of professionals, especially ESR (65.9%) and both parameters are used much less frequently (18.7%).20 In our study, the utilization rates were higher, 77.8% for ESR and 75.1% for CRP. According Donald et al., factors influencing the decision to request a laboratory test, in order of highest to lowest degree of importance are: clinical experience, evidence from the literature, learning as an intern or resident, the experience of other specialists and the economic impact; it also states that the majority of survey participants employed laboratory tests in the same way than other rheumatologists, which could be considered as one of the basic proposals the hypothesis of uncertainty, which refers to the low variability when there is agreement among clinicians about the value of a procedure.21 Furthermore, these authors analyze whether the request for laboratory tests is associated with several variables (geographic region, average number of patients per month, insurance and the existence of a laboratory), observing no differences between those using and not using laboratory tests, although in the RA group statistically significant differences were found between those using and not using laboratory tests in more than 50% of visits, since the latter professionals are more likely to have practiced in a university hospital while the distribution of other workplaces is similar between the two groups.20 In this sense, a teaching hospital is a factor of variability in the use of laboratory parameters, with significant variations in the use of rheumatoid factor, other laboratory tests and peripheral or axial X-rays, between rheumatologists rheumatology residents and non-teaching hospitals, with a slight increase in use by the formerprimeros.12 However, Henke et al. considered that differences in the style of the individual practitioner (tendency of suppliers to use procedures more or less frequently than the average), is the most important cause of variation in the use of these tests.22 In addition, Maravic et al. expose other factors contributing to the heterogeneity found between studies, such as the variability due to inadequate access to health care and/or health insurance or inadequate continuing medical education.23
In relation to pain assessment and evaluation of the activity, the eMAR II shows that the completion of a VAS score is not a widely used clinical follow-up procedure, the most common being the 28 joint count, as shown in Table 3 by comparing the values between the studies.19 Although Bellamy et al. showed higher percentages of use when monitoring treatment with NSAIDs (68%), second-line therapy with DMARDs (76%) and glucocorticoids (66%), which may be explained by the use of scales in clinical monitoring and is a variation in studies based on past practice or clinical trials.19 In this regard, Pincus et al. state that at a convention, experts were asked about the frequency in conducting counts of tender and swollen joints in relation to the number of routine visits of patients with RA (at any visit, from 1 to 24, 25 to 49, 50 to 74, more than 75% of visits and always) and found that the following percentages of joint counts per number of visits: 13%, 32%, 11%, 14%, 16% and 14%, respectively; as Bellamy et al. reported, the discrepancies can be explained by the type of study, as is done in an international convention rather than actual observations or record review clínicas.25 Also the discrepancy between the theoretical importance attributed to the use of quantitative measurements in the practice, expressed in a study by Singh et al., and based on the attitudes of the clinician in cervical spondylotic myelopathy, suggesting that these scales are underused or unsuitable for clinical practice and conclude that might require a new level of ease of use and better reflect clinical requirements.26 Thus, for 80% of rheumatologists participating in the Bellamy et al. study, the relevant features of the measurement procedures used in clinical practice are: simplicity, quick completion, easy scoring, reliability, validity and sensibility.19
Extra-articular manifestations of eMAR II can be framed with a study conducted in 15 countries, showing a variation in the prevalence of extra-articular disease with 15% (Netherlands, Italy) to 30% (Germany, Denmark, Poland, Great Britain), with 22.9% for Spain.27
Regarding the work disability, when compared with a cohort where the observation period was 9 (4–16) years, there were 37% of patients with work disabilities.28 The data recorded in the eMAR II (54.4%) are similar to eMAR I (49.1%).18 Work disability incidence in eMAR I in occupationally active patients with RA was 14.4 people per 100 patients in two years, while in eMAR II this result was 7.2%, although it should be noted that the working life details are only collected in a total of 460 clinical histories, so these results should be interpreted with some caution, since the efficiency in collecting the data was not entirely correct. First, it is quite common that the patient history does not collect such information, but disability pension collection has not been limited exclusively as a derivative of the disease under study, whereas other more or less disabling processes are included (Table 1). When interpreting the results of this study one must take into account its limitations. The duration of data collection was only 2 years and, therefore, the interpretation of results should be used with caution. Furthermore, it should be noted that the data cannot be easily removed from the patient history and that may be non-detectable in the written document, which affects the validity of results.4 It may be added that the differences listed above, found in the use of scales and scores in clinical practice or joint counts depending of the type of study, present variation according to studies carried out based on past practice or clinical trials,19,24 and as already stated in other studies quantitative indices are infrequently used in common clinical practice.29 In conclusion, we can summarize that, despite the existence of clinical practice guidelines for RA (GUIPCAR),30 eMAR II results show significant variability in some sections of the patient history, with frequent use of clinical evaluation parameters and joint counts, but less commonly pain assessment, disease activity, functional capacity and composite indices such as DAS-28. Such studies can detect the degree of compliance with recommended clinical practice guidelines and decrease the VCP.
FinancingThe Spanish Society of Rheumatology and Abbott laboratories supported the emAR II study.
Conflict of InterestThe authors declare no conflict of interest.
emAR II Study Group (Spanish Society of Rheumatology): J. Alegre, J.L. Alonso, M. Alvarez, B. Alvárez, A. Aragón, F.X. Arasa, M.J. Arias, J. Beltrán, J. Babío, C. Bohorquez, D. Boquet, S. Bustabad, A. Casado, J. Calvet, S. Castro, M.R. Colazo, E. Collantes, E. Cuende, N. Chozas, E. Delgado, D. de la Fuente, A. de Juanes, E. del Rincón, E. Enríquez, C. Escudero, L. Espadaler, P. Espino, A. Fernández, J. Fernández, L. Fernández, J. Fiter, P. Font, J. Galvez, A. Gallego, J. García, J. García, M.E. García, F. Gamero, E. Giménez, S. Gómez, B. González Álvarez, S. González, M. Granados, G. Iglesias, V. Irigoyen, F. Jimenez, E. Júdez, C. López, M. López, R. López, F.J. López-Longo, J. Maese, F.J. Manero-Ruiz, S. Manrique, I. Maries, C. Martínez, A. Martínez-Cristóbal, I. Mateo, J. Marzo, F. Medina, J. Medina, M. Medrano, P. Mesa, R. Miguélez, I. Monteagudo, C. Montilla, I. Moreno, M.L. Muñoz, A. Naranjo, R. Negueroles, M. Nolla, S. Ojeda, C. Ordás, S. Ordóñez, A.M. Ortíz, E. Pagán, A. Pecondón, S. Pérez Esteban, E. Pérez-Pampín, J.M. Pina, J.A. Piqueras, M.J. Pozuelo, V. Rios, N. Rivera, C. Rodríguez, J.M. Rodríguez, R. Roselló, M.J. Rubira, D. Ruiz, E. Saiz, M. Sánchez, T. Tinturé, C. Tornero, J. Tornero, E. Úcar, I. Ureña, C. Vázquez, R. Veroz, E. Vicente and J. Zubieta.
Please cite this article as: Maese J, et al. Estudio sobre el manejo de la artritis reumatoide en España (emAR II). Características clínicas de los pacientes. Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2012.02.011
See in Appendix 1.