To determine the clinical efficacy and safety of Leflunomide (LFN) 100mg/week compared to low dose Methotrexate (MTX) 10mg/week in a double-blind, randomized, controlled trial with 52 weeks of follow up in Rheumatoid Arthritis (RA) patients.
Patients and methodsPatients who met ARC1987 criteria for RA were included. All patients had medical records, including laboratory tests and hand X-rays. Clinical evaluations for improvement and ACR and EULAR response criteria were performed. Statistical analysis for independent's samples between both groups defined a P value of ≤.05. Safety was evaluated by comparing the proportion of adverse events (AE) registered.
ResultsOf the 90 patients screened, five were withdrawn and the remaining 85 patients were randomized: 43 LFN and 42 MTX. Sixty-three patients completed the study, 72% in the LFN group and 74.4% in the MTX group. ACR20 improvement criteria were achieved by LFN group in 90.3%, and in MTX 78.1% (P=.14) at week 52. EULAR improvement criteria applied at the end point showed a DAS28 score for the LFN group of 3.45, and for the MTX group was 3.67 (P=.43). Total withdrawals including loss during follow up, AE and lack of efficacy for each group was 12 patients in the LFN group, and 10 patients in the MTX group. Regarding safety, no serious AE of a life threatening nature were reported.
ConclusionsThese outcomes confirm that LFN 100mg/week offers an adequate and sustained improvement effect on the clinical manifestations of RA, similar to low dose treatment with MTX 10mg/every week after 52 weeks of follow up; it may be a good therapeutic option alone or in combination with other anti-rheumatic drugs.
Estudio clínico aleatorizado para determinar la eficacia y seguridad de leflunomida (LFN) 100mg/semana en artritis reumatoide (AR), comparado con dosis bajas de metotrexate (MTX) 10mg/semana a 52 semanas.
Pacientes y métodosSe incluyeron pacientes con criterios de AR activa (ACR1987). Fueron realizados estudios de laboratorio, radiografías de manos y determinaciones clinimétricas para establecer criterios de respuesta clínica de ACR y EULAR. El análisis estadístico se obtuvo a través de mejoría ACR20. La eficacia se estableció por análisis de ANOVA de muestras independientes entre ambos grupos (p≤0,05). La seguridad fue analizada con porcentaje de eventos adversos.
ResultadosDe 90 pacientes evaluados, 5 fueron eliminados; 85 aleatorizados e incluidos en 2 grupos: 43 (LFN) y 42 (MTX). Completaron el estudio con LFN el 72% y con MTX el 74,4%. El criterio de mejoría de ACR20 al final del estudio fue alcanzado para LFN en 90,3% y para MTX 78,1%, p=0,14. El valor DAS28 al final para LFN fue de 3,45, y para MTX de 3,67, no existiendo diferencias significativas (p=0,43). Los pacientes excluidos para LFN fueron 11, y 10 para MTX. La falla terapéutica se definió en 5,2% para LFN, y 12,1 en el caso de MTX. No se reportaron eventos adversos graves que pusieran en riesgo la vida de los pacientes.
ConclusionesLos resultados confirman que LFN usada en dosis semanales de 100mg, ofrece una adecuada y sostenida mejoría de las manifestaciones clínicas de AR, al compararlo con una dosis baja de MTX. Pudiendo ser una opción terapéutica en algunos pacientes como monoterapia o en combinación con otros antirreumáticos.
Leflunomide (LFN) is a non-biological disease-modifying antirheumatic drug (DMARD), an inhibitor of purine synthesis, which is indicated for the treatment of rheumatoid arthritis (RA). There are several published clinical studies that have demonstrated its benefit and safety, being considered equivalent to treatment with sulfasalazine (SFA) or methotrexate (MTX).1–3
One problem that prevails in the treatment of RA is the compliance (respecting prescription doses) and adherence (to maintain the treatment for a long period of time) to DMARD treatment, a difficult situation to achieve due to multiple factors such as are polypharmacy, adverse drug events and the high cost of treatment, especially in those patients who lack social security coverage, all of which makes it difficult to obtain good long-term clinical outcomes in routine clinical practice.
Seeking treatment alternatives that benefit the compliance and adherence of the treatments, as well as maintains the effectiveness of antirheumatic treatment, we developed an open descriptive study using LFN weekly doses of 100mg in active RA patients followed up to 6 months, who achieved clinical improvement according to criteria of the American College of Rheumatology (ACR), with no evidence of serious adverse events.4
In the present study, we describe the efficacy and safety results of patients treated in a randomized, comparative, double-blind trial of LFN given at a weekly dose of 100mg, compared with a fixed low dose of MTX 10mg/week with 52 weeks of follow up.
Patients and MethodsPatient PopulationPatients included in the study were adults who met the ACR19875 criteria for classification as active RA. Patients were enrolled from June 2004 to December 2007 from the outpatient clinic of RA. Active RA was defined for those patients who had at least 6 or more swollen (SJ) and painful (PJ) joints, morning stiffness greater than 30min and erythrocyte sedimentation rate (ESR) of 20mm/h or greater. Previous treatment with DMARDs should have been suspended at least one month prior to enrollment, and more than 3 months prior for LFN or MTX. Newly diagnosed patients without DMARD treatment were also included.
The use of prednisone or its equivalent was allowed with a regular dose not exceeding 10mg daily for the shortest possible time. Patients were excluded if a history of high alcohol consumption was present and pregnancy or a possibility thereof. Baseline laboratory studies requested for inclusion were: normal count of white blood cells, hemoglobin concentration greater than 12g/dl, albumin levels ≥3.5g/d, normal liver function tests and if female, negative pregnancy test.
Study ProtocolA randomized controlled trial with a 52-week follow up started in 2004 after approval by the local Committee for Research and Ethics with the registration number 11331-1200-209B-UEeI 322-2003 at the State and Municipalities Social Security Institute of Mexico (ISSEMyM), conducted under the guidelines of the International Declaration of Helsinki. The process of informed consent was required for all patients and in addition, females and patients of reproductive age were required to show confirmation of not being pregnant and the use of effective birth control during the development of protocol or until the doctor indicated.
All patients underwent complete medical history, physical examination, laboratory tests and radiographs of hands and feet, the latter for purposes of diagnostic classification. Clinimetric determinations were recorded, which included: 28 joint count (tender and swollen), patient (PGA) and physician (MDGA) global assessment on a visual analog scale (VAS 0–100mm), patient pain score (VAS 0–100mm), a validated functional physical limitation questionnaire for Spanish-speaking patients (HAQ-Di in Spanish)6; baseline laboratory studies required were erythrocyte sedimentation rate (ESR, Westergren), C-reactive protein, blood count and liver function tests. Clinical and laboratory tests were performed at the start of patient enrollment and monthly for 3 months, followed by visits every 2 months to complete the 52-week follow up. The X-ray studies of hands and feet were made only at the beginning of the protocol. All laboratory tests and imaging were performed at Toluca's ISSEMyM Medical Center, under standardized techniques validated according to international protocols of good clinical laboratory practice.
The primary study objective was to evaluate the clinical improvement of the disease according to ACR improvement criteria, with 20% improvement in swollen and tender joints and at least one of the following to determine ACR improvement: pain, global assessment of disease by the patient and the physician, HAQ-DI and acute phase reactants. Also included were additional ACR 50 and 707 results, DAS 28, the criteria for disease activity and improvement of the European League Against Rheumatism (EULAR) at each visit and at the end of the study, EULAR8 referral criteria and recording of treatment discontinuation due to adverse events.
The criteria for discontinuation of patients in the study were applied to all those who did not achieve ACR 20 improvement at week 16, or if the patient had serious adverse events (SAE), which would require unblinding the drug safety status. For patients who had elevation of transaminases in a recurring manner, we discontinued them from the study with the following criteria: values greater than 2.5 times normal transaminase levels (AST and ALT) for 2 consecutive months.
Assigning Treatment GroupsPatients were randomized into 2 blocks using a table of random numbers, without the intervention of the research group (1:1): the target for the LFN group and a control group of MTX. For the target LFN group, a loading dose of 100mg/day for 3 consecutive days was given, based on the average half-life of the drug, and administered at a weekly dose of 100mg. For the MTX group, a fixed low dose of 10mg weekly was administered; for both groups, placebos were administered in numerical form in an equivalent manner to achieve the blinding of patients and medical researchers.
Statistical AnalysisThe primary objective of the study was to compare the efficacy and safety of a weekly dose of 100LFN mg compared to the effect achieved with low dose of MTX 10mg weekly. The efficacy was measured by ACR 20 improvement criteria as a study endpoint at 52 weeks of treatment. Variables also included were ACR 50 and 70 improvement, EULAR improvement criteria, and an independent evaluation of the ESR and HAQ-Di variables.
Efficacy was established by independent-samples ANOVA between groups at 8, 24 and 52 weeks. Data were considered statistically significant if P≤.05. Safety was analyzed according to the percentage of adverse events reported in each group.
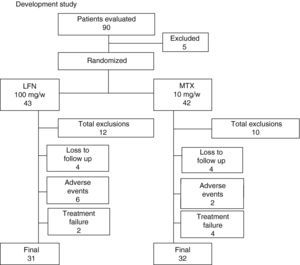
ResultsOf the 90 patients evaluated for study entry, 5 were excluded, and the 85 remaining were randomized into 2 groups as follows: a group of 43 patients were assigned to LFN and 42 to MTX (Fig. 1). Both groups of patients were assessed at least once during follow up from the baseline visit. The demographics and disease characteristics were similar for both groups (Table 1). Three patients were treated with DMARDs prior to enrollment, 2 for the LFN group. One of them who received LFN 20mg/day and hydroxychloroquine for 2 months discontinued treatment for 7 months before being randomized to the LFN group. A second patient, with an irregular treatment, took MTX for a month, 3 months after being randomized to the LFN group. Finally, in a patient receiving conventional LFN, diffuse alopecia developed after 2½ months and treatment was suspended; the patient was sent to our hospital and included in pre-randomization, after no treatment was given for 3 months, to the MTX group. Sixty-three patients completed 52 weeks of treatment, 31 in the LFN (72%) and 32 in the MTX group (74.4%). Early discontinuation of patients at week 16 occurred more often in the LFN than in the MTX group (19.4 vs 5%), respectively. At the end of the study, the total of patients who left were 21, either by loss to follow up or adverse events. Twelve cases occurred (27.9%) in the LFN and 10 patients (23.8%) in the MTX group. Discontinuation due to lack of efficacy was found in 2 patients in the LFN group (5.2%) and in 4 cases with MTX (12.1%) (Fig. 1).
This Flowchart shows the progression of the patients evaluated and included in the study. Patients were excluded if they did not meet inclusion criteria, two were lost to follow-up and one withdrew informed consent before randomization. Twelve patients withdrew from the LFN group and 10 from the MTX. The reason for exclusion is explained in detail in the text. End of the study was achieved in 74% for both groups, 31 patients in the LFN and 32 in the MTX.
Disease Characteristics and Demographic Data.
| Leflunomide Group±SD | Methotrexate Group±SD | Pa | |
| Number of patients (No.) | 43 | 42 | – |
| Age, years | 42.8 (±11.7) | 42.1 (±10.8) | .76a |
| Female, % | 88.3 | 85.7 | .56a |
| Duration of the disease, months | 25.2 (±6.8) | 20.9 (±3.5) | .57a |
| Tender joint count, 0–28 | 11.1 (±5.1) | 11.5 (±6.1) | .74a |
| Swollen joint count, 0–28 | 8.9 (±4.8) | 7.5 (±4.9) | .17a |
| Global disease score by the patient (activity), 0–100mm, VAS&. | 43.1 (±15.1) | 44.5 (±15.1) | .67a |
| Global disease score by the physician (activity), 0–100mm, VAS& | 52.2 (±12.3) | 52.1 (±15.2) | .95a |
| Pain score, 0–100mm, WAS& | 70.7 (±26.2) | 70.6 (±20.5) | .98a |
| HAQ-Di | 0.96 (±0.09) | 0.83 (±0.07) | .27a |
| DAS 28 | 5.8 (±0.96) | 5.6 (±0.88) | .24a |
| Theumatoid factor presence, % | 41 (±95.3) | 39 (±90.7) | .53a |
| Erythrosedimentation rate, mm/h | 35.4 (±13.5) | 30.2 (±15.0) | .10a |
| Prior DMARD treatment | 2 (4.6%) | 1 (2.3%) |
SD: standard deviation; VAS: visual analog scale; DMARD: disease modifying antirheumatic drugs.
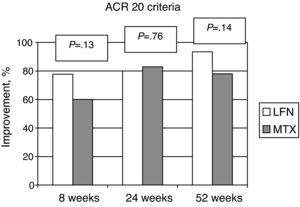
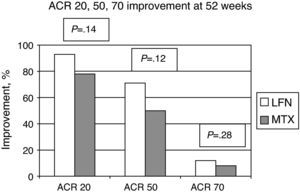
The ACR improvement criteria were assessed at weeks 8, 24, and 52. In patients assigned to LFN, 28 (80%) achieved ACR 20 at week 24 and in 29 cases (93.5%) at week 52. For the MTX group the results showed that 30 patients (83%) achieved ACR 20 at week 24 and 25 (78.1%) at week 52; comparing the two groups, there was no statistically significant difference (Fig. 2). Evaluating the results of the study end point for ACR 50 and ACR 70 we found no significant differences by comparing the groups for these variables (Fig. 3).
The independent variables were evaluated and the results of the HAQ-Di at baseline scored 0.96 for the LFN group and 0.83 for MTX (P=.27). The final evaluation of study data showed a score of 0.23 for LFN and 0.39 for MTX, with a reduction of 0.7 and 0.43, respectively for each study group, with a marginal difference when evaluating this data (P=.05) in the LFN group.
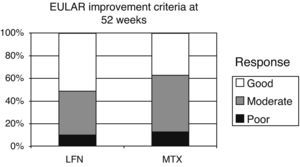
EULAR criteria for improvement and remission were evaluated at week 52 of the study. Initial DAS 28 results of the LFN group were 5.83 and 3.45 at 52 weeks (2.38 reduction points). For the MTX group a baseline score of 5.60 was seen, 3.67 at study end, with a net reduction of 1.93 points. There were also no statistically significant differences when comparing results between the two groups (P=.43). The standard cutoffs to define improvement in EULAR DAS 28 were as follows: <3.2 points=good response, from 3.2 to 5.1 moderate response >5.1 points no response (Fig. 4). Applying the EULAR remission criteria (<2.6 points), 11 patients of both groups met this criterion.
SafetySerious adverse events were considered by investigators in 9 cases, 2 dermatological reactions occurred in patients in the LFN group, one of them developing severe rash, another erythema multiforme on the trunk. Six patients had elevated liver enzymes 2.5 times above the normal range, four of them received LFN and 2 belonged to the MTX group and they were all withdrawn from the study (Fig. 1).
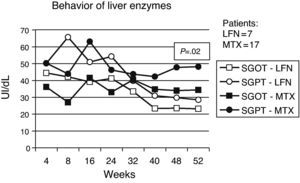
Less important events recorded for both groups included vasculitis, pruritus, alopecia, and headache. Presence of infections was observed in both groups, with a slightly higher percentage in patients with MTX treatment (Table 2). Gastrointestinal adverse events (GI) are described in Table 3, where episodes of diarrhea were more often present in the LNF group. The data recorded regarding alterations in liver function tests were as follows: 7 and 17 cases had elevated liver enzymes in the LFN and MTX groups. Four (9.3%) in the LFN group remained >2.5 times the normal range, so patients were withdrawn from the study. In 3 other patients, despite the high values referred to above, these returned to normal levels during the study. For the MTX group, 2 patients had elevations for which they were eliminated, another 7 returned to normal baseline levels without recurrence at study end; there were no statistically significant differences when comparing both groups (P=.02) (Fig. 5). Three cases of hypertension were detected, 2 in the LFN and one in the MTX group for both groups and classified as a minor event. Finally hematologic abnormalities documented in the LFN group consisted of leukopenia in 4 patients, 2 with anemia and one with thrombocytopenia; in the case of MTX there were 2 cases of leukopenia, 2 with anemia and one with mild thrombocytopenia.
Infectious Diseases Registered.
| LFN=43 | MTX=42 | |||
| No. | (%) | No. | (%) | |
| Upper respiratory tract infections | 6 | 13.9 | 12 | 28.5 |
| Urinary infection | 3 | 6.9 | 3 | 7.1 |
| Gastroenteritis | 1 | 2.3 | 1 | 2.4 |
| Herpes zoster | 0 | 0 | 1 | 2.4 |
| Vulvovaginitis | 1 | 2.3 | 3 | 7.1 |
A greater number of upper respiratory tract infections were seen in the MTX than in the LFN group.
Non-liver Gastrointestinal Effects.
| LFN=43 | MTX=42 | |||
| No. | (%) | No. | (%) | |
| Gastritis | 12 | 27.9 | 11 | 26.1 |
| Diarrhea | 9 | 20.9 | 1 | 2.3 |
| Abdominal Distension | 2 | 4.6 | 6 | 14.2 |
| Nausea | 3 | 6.9 | 6 | 14.2 |
| Other | 2 | 4.6 | 1 | 2.3 |
Gastrointestinal events such as gastritis and diarrhea were more frequently reported in the LFN than in the MTX group, as seen in the literature; however, abdominal distension was more common in the MTX group.
There were no adverse events that would jeopardize the lives of patients in any of the 2 groups.
DiscussionIn daily practice, rheumatologists have a need for RA treatment regimens that are effective and safe, in addition to being flexible in their administration, in order to maintain adherence and compliance to treatment and thus achieve the goals and objectives of clinical improvement or remission of disease.9
LFN is a non-biological DMARDs belonging to the isoxazole class; after administration it is rapidly converted to its active metabolite A77 1726; this metabolite induces its therapeutic effect by inhibiting the enzyme dihydrorotate dehydrogenase. This is an important key enzyme in pyrimidine de novo production in T lymphocytes. This molecule has a long plasma life of about 2 weeks (14–18 days).10
Published studies indicate that the ACR 20 improvement criteria in patients with RA treated with MTX monotherapy ranges from 40% to 60% at 6 and 12 months of follow up.11 On the other hand, it is well known that treatment of RA patients at doses of 20 LFN mg/day has shown benefit in clinical response similar to MTX and other DMARDs as SSZ.11–13 Jakez-Ocampo et al. published a pilot study using LFN as an open treatment at 100mg weekly in patients with refractory RA.14 This study included 16 patients, 8 of them in treatment with LFN 100mg/week and another 8 with the regular dose of 20mg daily followed by a period of one year. The base treatment of patients was not changed, including at least the combination of 2 or 3 DMARDs in association with different doses of steroids. The results showed benefits in the initial treatment group of LFN 20mg/day; however, at the end of the study, no statistically significant differences between the 2 groups, including the development of ACR 20 improvement, was seen. Minor events were reported more frequently in the LFN 20mg/day group. A second study was conducted by the same authors,15 this time in 3 groups with a diagnosis of early RA (less than one year since onset). Thirty patients were divided into 3 groups: the first group of 10 patients treated with LFN 100mg/week, the second group of 10 patients treated with LFN 20mg/day and a third group treated with MTX at a dose of 7.5–15mg/week, with a one year of follow up. By the eighth week of the study, response was observed in all 3 groups, noting again the fastest response in the LFN20mg/day group compared to LFN 100/week and MTX/week with a P=.001 and P=.03, respectively. The variables assessed at the end of the study showed no significant differences in any of the 3 groups. In relation, the presence of adverse events was more frequent in the LFN 20mg/day and MTX/week groups compared to LFN 100mg/week.
In addition, our group previously performed an open 6 month clinical trial at LFN weekly dose of 100mg in patients with active RA.4 Fifty patients were enrolled in the study, starting treatment with a loading dose of 100mg/day for 3 days followed by a weekly dose of 100LFN mg for a period of 6 months. After 12 weeks, 75% of patients had achieved ACR 20 improvement response and 58% achieved an ACR 50. At study end, 74% achieved ACR 20, 64% of patients achieved ACR 50 and ACR 70 28% improvements.
Adverse events reported in the study ranged from 2% to 16%, which included headache, rash, hair loss, elevated liver enzymes and diarrhea. It was concluded that clinical benefit in response to a weekly regimen of 100mg of LFN is associated with minor adverse events already reported previously.
The results obtained in this study show that both drugs, at doses lower than those recommended, help compliance and adherence to treatment in a very acceptable percentage, emphasizing that the low dose of MTX of 10mg/week is only presently recommended at baseline, increasing it if tolerated quickly and in a stepwise fashion.16 We observed that at week 52, retention of patients was 31 patients (72%) and 32 cases (76%) for the LFN and MTX groups, respectively, with an overall retention of 74%, a situation that differs from reports by other authors, which present more than a 50% loss in studies to LFN at a standard17 dose; a similar number is reported in patients with long-term treatment with MTX.18
The results of ACR improvement, HAQ-DI and ESR did not differ between groups, stressing that the dose of MTX used is currently considered suboptimal and not comparable for assessment of the efficacy of MTX in this study, as the current recommendations of EULAR point out, where a rapid increase up to 20 or 25mg/week is indicated in order to reduce clinical activity. Of patients who completed the study in the LFN group, 28 achieved an ACR 20 response (90.3%) at week 52 (Fig. 2); however, applying the calculation of patients intended to treat (ITT), the ACR 20 response was 67.4%. Two patients were eliminated from the LFN group for not achieving ACR 20 improvement, compared to MTX where 4 cases did not achieve it.
Regarding adverse events, those seen in the LFN group were similar to those reported in the literature, affecting the skin with erythematous urticaria, alopecia and diarrhea. Liver toxicity was apparently lower in the LFN group, and only one patient remained with persistent enzyme elevation; in this case we ruled out viral hepatitis, and only found fatty liver by conventional ultrasound. The few non-serious adverse events identified were probably related to the low dose of LFN employed.11
We conclude that the weekly dose of 100mg of LFN provides an adequate and sustained response in patients who respond to this drug, allowing for greater adhesion and compliance than reported in the literature for conventional treatment, besides apparently showing fewer reported adverse events compared with the recommended standard dose. It currently constitutes the loading dose in common practice and is not commonly used as described here; therefore, unfortunately some countries have recalled tablets with LFN 100mg. This scheme also opens the possibility of its use as monotherapy or in combination with other DMARDs, including MTX as an attractive option avoiding polypharmacy.19 Moreover, the weekly dose of 100LFN mg/week represents a savings for patients, using a lower dose of the drug while maintaining its effectiveness and this situation applies only in countries where there is no health system that allows full coverage of the population.
Finally, we emphasize that the lack of efficacy observed in patients in the MTX group could be a reflection of the low dose used for the purposes of this study, but by no means constitute a recommendation by the authors for use in daily clinical practice.
Studies with larger populations and longer durations will ratify the results we obtained in this study.
FinancingThis study or the researchers had no financial relationship with the pharmaceutical industry. The drugs employed were obtained through the institute (ISSEMyM) where research was carried out.
Conflict of InterestThe authors have no conflict of interest to declare.
To Dr. Eduardo Brea Andrés, for his review of the methodology and result analysis; and to Dr. Rodrigo Suárez Otero for his review and suggestions.
Please cite this article as: Jaimes-Hernández J, et al. Eficacia de leflunomida 100mg semanales comparado con dosis bajas de metotrexate en pacientes con artritis reumatoide activa. Estudio clínico doble ciego aleatorizado. Reumatol Clin. 2012;8:243–9.