Anti-malarials are widely used in the treatment of autoimmune diseases, especially hydroxychloroquine (HCQ). Mepacrine is used in cases of contraindication or poor response to HCQ, and is sometimes used in combination with it.1 Its use can cause itching and dermatological conditions such as rashes and hyperpigmentation, usually mild.2 Hyperpigmentation can affect the skin and mucous membranes.3,4 HCQ induces grayish or black skin lesions, and mepacrine, yellowish discoloration.5 Nail pigmentation caused by these drugs is much less well known.2
We present the case of a patient treated with mepacrine, which caused yellowish skin discoloration and bluish pigmentation of the fingernails and toenails.
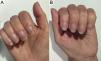
A 50-year-old woman with a history of Sjögren's syndrome and systemic lupus erythematosus with lupus nephropathy was advised against treatment with HCQ by the Ophthalmology Department due to miliary drusen in the retina and early macular degeneration. She began treatment with mepacrine, 100 mg/day for one year and then 400 mg/week, and presented with yellowish skin pigmentation (Fig. 1). After four years of treatment, she noticed the progressive appearance of horizontal blue-gray bands on her fingernails (Fig. 2A and B, arrows) and on several toenails.
Nail pigmentation due to antimalarials has an unknown incidence, which is probably underestimated, and could range from .1% to 37.4%2,6 for HCQ. Painless, longitudinal, brownish-black melanonychia lesions usually appear on the fingernails and toenails. When only one nail is affected, a differential diagnosis with melanoma should be considered. Melanonychia is not considered dose-dependent, although it generally occurs in patients with a higher cumulative dose.3 Discontinuing antimalarials can cause the hyperpigmentation to disappear, but it does not always completely resolve.3
Skin and nail pigmentation due to mepacrine is a non-toxic side effect that should be known by both the patient and their treating physicians to avoid inappropriate withdrawal of this drug or the performance of unnecessary complementary tests.
FundingNone of the authors received any kind of external funding.
The authors have no conflict of interests to declare.