Hydroxychloroquine (HCQ) is the first-line treatment for systemic lupus erythematosus (SLE); however, there is heterogeneity in its clinical use. This consensus aims to bridge the gap in SLE treatment by providing practical and valuable recommendations for health professionals.
MethodsThe methodology used is based on a systematic literature review and a nominal group technique (NGT). A ten-member scientific committee formulated eight clinically relevant questions. First, a systematic review was conducted to identify the available evidence, which the scientific committee evaluated to developed recommendations based on their expertise, achieving consensus through NGT.
Results1673 titles and abstracts were screened, and 43 studies were included for meeting the inclusion criteria. The scientific committee established 11 recommendations for HCQ use in initiation, maintenance, and monitoring, considering benefits and potential adverse effects of HCQ. Unanimous agreement was achieved on all recommendations.
ConclusionsThe available evidence supports HCQ's effectiveness and safety for SLE. Individualized assessment of the initial HCQ dose is important, especially in situations requiring dose reduction or discontinuation. This risk–benefit assessment, specifically focusing on the balance between retinal toxicity and the risk of SLE relapse, should guide decisions regarding medication withdrawal, considering disease activity, risk factors, and HCQ potential benefits. Close monitoring is essential for optimal disease management and minimize potential risks, such as QT prolongation or retinal toxicity.
La hidroxicloroquina (HCQ) es el tratamiento de primera línea para el lupus eritematoso sistémico (LES); sin embargo, existe heterogeneidad en su uso clínico. Este consenso tiene como objetivo superar la disparidad en el tratamiento del LES al proporcionar recomendaciones prácticas y valiosas para los profesionales sanitarios.
MétodosLa metodología utilizada se basa en una revisión sistemática de la literatura y en el uso de la técnica de grupo nominal (NGT). Un comité científico formado por 10 expertos formuló 8 preguntas clínicamente relevantes. En primer lugar, se realizó una revisión sistemática para identificar la evidencia disponible. Posteriormente, el comité científico desarrolló unas recomendaciones basadas en la evidencia y en su experiencia clínica, consiguiendo un consenso a través de la NGT.
ResultadosSe examinaron 1.673 títulos y abstracts y se incluyeron 43 estudios que cumplían con los criterios de inclusión. El comité científico estableció 11 recomendaciones para el uso de la HCQ en el inicio, en el mantenimiento y en el seguimiento, considerando sus beneficios y posibles efectos adversos. Todas las recomendaciones obtuvieron un acuerdo unánime.
ConclusionesLa evidencia disponible respalda la efectividad y seguridad de la HCQ para el LES. La evaluación individualizada de la dosis inicial de HCQ es importante, especialmente en situaciones que requieren una reducción o interrupción de la dosis. La evaluación de riesgo/beneficio, centrándose específicamente en el equilibrio entre la toxicidad retiniana y el riesgo de recaída del LES, debería guiar las decisiones relacionadas con la retirada de la medicación, teniendo en cuenta la actividad de la enfermedad, los factores de riesgo y los posibles beneficios de la HCQ. La monitorización estrecha es esencial para un manejo óptimo de la enfermedad, y para minimizar riesgos potenciales, como la prolongación del intervalo QT o la toxicidad retiniana.
Systemic lupus erythematosus (SLE) is a chronic rheumatic autoimmune disease that affects multiple organs and systems. In Spain, its estimated prevalence is 210 cases per 100,000 inhabitants.1 The disease is characterized by the production of autoantibodies that target healthy tissues, leading to chronic inflammation.2 Clinical manifestations and disease course are heterogeneous among affected individuals.1
Hydroxychloroquine (HCQ) is an antimalarial drug with immunomodulatory and anti-inflammatory properties,3 is one of the primary treatments used in SLE. Studies have shown that HCQ reduces disease activity and enhances long-term outcomes, including increasing survival, reduced risk of flares and organ damage.4
The current recommendations by the European Alliance of Associations for Rheumatology (EULAR) suggest using HCQ as the first pivotal therapy for all SLE patients without contraindications, with an emphasis on monitoring both the dose and retinal toxicity.5 Studies suggest that adjusting the HCQ dosage in accordance with the American Academy of Ophthalmology (AAO) guidelines have a significant impact on the short-term and mid-term outcomes on patients with SLE.3,6 However, reducing or discontinuing HCQ may trigger disease exacerbations and, at the same time, lost many other benefices associated to its use.7 It is, therefore, imperative not only to evaluate the risk of flares associated with HCQ withdrawal but also to closely monitor patients’ treatment to mitigate the risk of irreversible side effects.8
Despite its wide therapeutic use and the existing recommendations on HCQ for the control of adverse effects, there is unwarranted variability in clinical practice in the treatment of SLE because the definition of an adequate daily dose and the optimization of the pharmacological management of SLE with HCQ continues to be controversial.9
This consensus aims to provide practical recommendations based on the currently available evidence and guided by the opinion of the participating experts on the use of HCQ in the clinical management of SLE, that can be used as a reference for health professionals involved in the treatment of this pathology.
MethodsThe current document is the result of a systematic literature review and a nominal group technique (NGT) with the scientific committee. This scientific committee comprised ten medical specialists, including eight rheumatologists, one ophthalmologist, and one pediatric rheumatologist from different Spanish centers with expertise in managing SLE patients. The methodology of this consensus has been endorsed by the Spanish Society of Pediatric Rheumatology (SERPE) and the Spanish Society of Ophthalmology (SEO).
A research protocol study was conducted to define objectives and methodology. From three proposed topics (treatment initiation, treatment maintenance, and clinical monitoring), eight clinical questions were formulated using the PICO question format (Patient, Intervention, Comparison, Outcomes).10
Systematic literature reviewA systematic literature review was carried out following the recommendations of the PRISMA.11 To address specific questions, a search strategy was developed. Initially, searches were conducted in databases such as Medline, Ovid, GIN, and the National Guideline Clearinghouse. Subsequently, a systematic electronic search of Medline and Epistemonikos was performed, covering the inception of the databases up to January 4, 2023.
The evidence underwent the prioritization of publication types to address clinically relevant questions effectively. Two reviewers independently evaluated references based on predefined inclusion and exclusion criteria (see Table S1). Selected references underwent a full-text screening to confirm eligibility. Any discrepancies were resolved through discussion or consultation with a third reviewer. Data extraction and verification were carried out by two reviewers independently, with discrepancies resolved by a third reviewer.
Grade of recommendation and level of evidenceThe Scottish Intercollegiate Guidelines Network (SIGN) approach was used to establish the level of evidence and grade of recommendations.12
Nominal group techniqueAfter drafting preliminary recommendations based on available evidence, a meeting the scientific committee using the NGT for equitable participation.13,14
Members provided individual assessment of the recommendations in a pre-work phase. During the meeting, the recommendations were systematically reviewed, modified in real-time for clarity and discussed for consensus. Anonymous voting was conducted generating agreement percentages for each one. Consensus recommendations are included in this document.
Statistical analysisThe study used a questionnaire with binary responses (Yes/No) to calculate agreement and disagreement percentages for each item. Consensus was defined as or higher agreement, and 100% agreement was defined as unanimity. A percentage between 66% and 79% was considered a discrepancy. Any recommendation with less 66% agreement was rejected.
The methodology of this consensus has been endorsed by the Spanish Society of Pediatric Rheumatology (SERPE) and the Spanish Society of Ophthalmology (SEO).
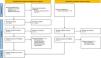
ResultsSystematic literature reviewWe identified 1673 publications in selected databases. After removing duplicates (n=121), we manually screened 1552 publications. Based on titles and abstracts, 1502 publications were excluded. Among the remaining 50 reports, 15 were excluded. Additionally, 13 publications were identified through citation searching. After assessing these 13 studies, 5 were excluded due to design issues. Ultimately, 43 records were included in the review. Fig. 1 shows the study's inclusion process.
Consensus process resultsAll recommendations achieved 100% agreement, thus unanimous consensus. Table 1 shows final recommendations.
Final recommendations.
| Recommendation | Grade of recommendation |
|---|---|
| Topic 1: Start of the treatment | |
| 1.1 It is recommended HCQ for all patients with SLE, unless contraindicated, at a dose not exceeding 5mg/kg/day (actual body weight). | C |
| 1.2 It is recommended dose adjustment (50% reduction) from baseline in patients with a glomerular filtration rate <30ml/min. | C |
| Topic 2: Treatment maintenance | |
| 2.1 It is recommended to reduce the dose of HCQ in patients with SLE when there is evidence of adverse reactions. | C |
| 2.2 In patients in prolonged remission, the possible reduction can be evaluated by assessing the risk–benefit ratio. | C |
| 3.1 The total discontinuation of HCQ is not recommended unless patients experience severe or intolerable adverse effects that do not respond to dose reduction. | C |
| Topic 3: Clinical and therapeutic monitoring | |
| 4.1 It is recommended to perform at least one baseline eye examination during the first 6–12 months of HCQ treatment in patients with SLE. | D |
| 5.1. It is recommended to perform spectral-domain OCT (SD-OCT) as the main test in each ophthalmologic examination. In cases with suspected toxicity, it is recommended to confirm functional involvement with visual fields. Other techniques such as autofluorescence or multifocal ERG (electroretinogram) should be reserved for doubtful cases, at the ophthalmologist's discretion. | D |
| 5.2 It is recommended to perform annual ophthalmological monitoring from the fifth year of HCQ use, except in patients with risk factors* for early toxicity in which check-up would be performed annually. | D |
| 6.1 It is recommended to perform a baseline ECG in patients with risk factors for prolongation of QT interval. | B |
| 7.1. No specific plasma determinations are considered necessary for toxicity monitoring. Given the absence of sufficient evidence at present, the use of HCQ plasma levels for toxicity monitoring or dose adjustment is not recommended in daily clinical practice. | D |
| 8.1. It is recommended to focus on the clinical visit, asking the patient about adherence and verifying it through electronic prescription as most feasible strategy to monitoring adherence. | C |
Question 1. What should be the starting dose of HCQ treatment in patients with SLE?
Evidence for the recommendation:
EULAR guidelines recommend HCQ for all patients with SLE, with an initial prescription dose not exceeding 5mg/kg/day (actual body weight).5 Regarding the pediatric population, HCQ toxicity is rare in children with SLE,15 however, despite the lack of studies in children with SLE, there are studies with juvenile idiopathic arthritis in which they indicate that doses up to 6mg/kg/day are safe to use in this population.16
Level of evidence 3
Recommendation:
- 1.1.
It is recommended HCQ for all patients with SLE, unless contraindicated, at a dose not exceeding 5mg/kg/day (actual body weight). Although individualized according to the risk of flare.
- 1.2.
It is recommended dose adjustment (50% reduction) from baseline in patients with a glomerular filtration rate <30ml/min.
Grade of recommendation C
Comment: In clinical practice, physicians often initiate treatment with a higher dose of 400mg in young patients weighing 50kg or less with severe symptoms such as nephritis, pericarditis, etc. This exceeds the recommended 5mg/kg dose proposed by EULAR. While it is acknowledged that this practice may lack a solid scientific foundation, it is a widely accepted approach due to its perceived safety.
Topic 2: Treatment maintenanceQuestion 2. In what situations is it recommended to reduce the dose of HCQ in patients with SLE?
Evidence for the recommendation:
2019 EULAR recommendations suggest considering HCQ dose reduction in SLE patients with prolonged remission and/or adverse reactions to HCQ, although no studies have formally addressed this strategy.5
An international prospective study from Systemic Lupus International Collaborating Clinics (SLICC) cohort, conducted from 1999 to 2019, revealed a significantly increased risk of SLE flares after HCQ tapering or discontinuation. Notably, this study is the first to report heightened disease activity associated with decreased HCQ doses in newly diagnosed SLE patients.17
For the pediatric population, if drug-related side effects occur, treatment options should be reassessed and switched if necessary.16
Level of evidence 2+
Recommendation:
- 2.1.
It is recommended to reduce the dose of HCQ in patients with SLE when there is evidence of adverse reactions.
- 2.2.
In patients in prolonged remission, the possible reduction can be evaluated by assessing the risk–benefit ratio.
Grade of recommendation C
Question 3. In what situations is the withdrawal of HCQ treatment recommended in patients with SLE?
Evidence for the recommendation:
HCQ is one of the pivotal treatments for SLE for disease activity control, prevention of organ damage and specific comorbidities. However, despite its good safety profile, long-term use may lead to adverse effects. This situation raises the possibility of discontinuing the drug to minimize long-term exposure. Nevertheless, there is limited evidence regarding the progression of disease activity and damage following the discontinuation of HCQ treatment.18
A larger study compared patients who continued HCQ with those who discontinued it, finding increased SLE flares in those with less than 1 year of prior HCQ use.18
In 407 patients from the SLICC inception multicentre cohort, previously mentioned, in whom HCQ was discontinued, the risk of SLE flares was higher, even patients in a low disease activity state or in remission at the initial assessment, although their definition of flare did not distinguish between mild and moderate or severe cases.17
Level of evidence 2+
Recommendation:
- 3.1.
The total discontinuation of HCQ is not recommended unless patients experience severe or intolerable adverse effects that do not respond to dose reduction.
Grade of recommendation C
Topic 3: Clinical and therapeutic monitoringQuestion 4. Is an ophthalmological evaluation indicated at the beginning of HCQ treatment in patients with SLE?
Evidence for the recommendation:
Two studies delved into the relationship between HCQ usage and the risk of retinopathy development. Both studies emphasize the significance of routine ophthalmic monitoring for individuals receiving HCQ therapy. One study establishes a clear dose-response relationship. Furthermore, the second study highlights a substantially increased prevalence of HCQ/CQ-induced retinopathy in SLE patients.19,20
Both the AAO in 2016 and The Royal College of Ophthalmologists in 2018 recommended baseline ophthalmologic examinations for patients starting long-term hydroxychloroquine (HCQ) or chloroquine (CQ) therapy.21,22
However, in 2020 a revision was published and baseline testing for new initiators of HCQ or CQ is no longer recommended. This amendment is supported by recent evidence of a low rate of drug discontinuation as a result of baseline testing (less than 4%).23
For pediatric population, the European initiative SHARE (Single Hub and Access point for pediatric Rheumatology in Europe) recommends ophthalmologic evaluations before starting HCQ treatment in children with SLE, with annual follow-up exams and dosage adjustments for patients with renal or hepatic insufficiency and abnormal body weight.16
Level of evidence 2+
Recommendation:
- 4.1.
It is recommended to perform at least one baseline eye examination during the first 6–12 months of HCQ treatment in patients with SLE.
Grade of recommendation D
Question 5. What are the recommended tests and frequency for assessing retinal toxicity in patients with SLE receiving HCQ treatment?
Evidence for the recommendation:
The 2016 AAO recommendations, suggest annual reviews with OCT as a standard test, and periodic visual fields.21
The joint statement of the AAO, dermatology and rheumatology also indicate the OCT as the mainstays of early detection available as an objective test that generates high resolution “cross-sections” of the retina to show individual cell layers and potential regions of thinning.7
In a retrospective case–control study of patients who had used HCQ for at least 5 years, it was documented that the risk of HCQ-induced retinopathy is low when patients receive appropriate dosing and are monitored with recommended screening tests. The recommended tests include a comprehensive eye exam with automated visual field testing and spectral-domain optical coherence tomography (SD-OCT) to assess macular thickness.24
The evidence emphasizes the importance of ophthalmology monitoring and formal assessment of cases of potential toxicity by a retinal specialist, especially in patients on HCQ for more than 10 years, those needing higher doses, and those of older age at SLE diagnosis.25
Level of evidence 4
Recommendation:
- 5.1.
It is recommended to perform spectral-domain OCT (SD-OCT) as the main test in each ophthalmologic examination. In cases with suspected toxicity, it is recommended to confirm functional involvement with visual fields. Other techniques such as autofluorescence or multifocal ERG (electroretinogram) should be reserved for doubtful cases, at the ophthalmologist's discretion.
- 5.2.
It is recommended to perform annual ophthalmological monitoring from the fifth year of HCQ use, except in patients with risk factors* for early toxicity in which check-up would be performed annually.
Grade of recommendation D
*Retinopathy risk factors5,19,25: higher HCQ dose (daily and cumulative dose), treatment duration, kidney disease, concurrent tamoxifen therapy, previous ophthalmologic disease (macular degeneration, etc.).Table S2from supplementary material.
Question 6. Is there a need for any testing (pre- or on-treatment) for possible cardiac toxicity? What tests are recommended for cardiac toxicity in SLE patients on HCQ therapy, and how often?
Evidence for the recommendation:
The evidence suggests that patients with SLE have a higher cardiovascular risk, but also heart rate and QT interval can be affected by HCQ.26 The antiatherogenic and thromboprotective properties may reduce major adverse cardiovascular events such as cardiac failure, cardiovascular death, and ischemic stroke in SLE patients.27 However, these drugs may also interfere with cardiac ion channels and influence in the prolongation of QT interval, cardiac arrhythmias and cardiomyopathy.28,29
To evaluate the impact of HCQ on QTc intervals in SLE patients, two cohorts were analyzed. Cohort 1, a retrospective review of 90 SLE patients using electronic medical records, showed no significant differences in mean QTc between HCQ-treated and untreated patients, nor did it reveal variations based on chronic kidney disease (CKD) status. Moreover, cohort 2, a prospective study of 84 SLE patients prescribed HCQ, found that HCQ blood levels did not correlate with QTc interval, regardless of dosage, duration of exposure, CKD, or underlying cardiac abnormalities. This is the first study based on measured blood levels demonstrating the absence of a clinically significant increase in QTc levels in SLE patients chronically treated with HCQ.28
An analysis of over thirteen million adverse event reports from the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System, HCQ was shown to significantly increase cardiac adverse events.30 Conversely, a systematic review found that, except for a few case reports of nonfatal adverse events, HCQ is actually consistently associated with a lower incidence of cardiac adverse events.31
Level of evidence 1+
Recommendation:
- 6.1.
It is recommended to perform a baseline ECG only in patients with risk factors* for prolongation of QT interval.
Grade of recommendation B
*Risk factors for prolongation of QT interval32,33: concomitant use of macrolide antibiotics, smoking, nephritis, longer disease duration, older age at diagnosis.Table S3from supplementary material.
Question 7. What analytical determinations are recommended to monitor HCQ toxicity in patients with SLE?
Evidence for the recommendation:
Various methods can be employed for monitoring HCQ levels in the blood and detecting HCQ-induced liver and kidney issues.34,35 Lenfant et al. discovered that higher cumulative doses, longer treatment durations, and elevated HCQ blood levels were risk factors for HCQ-induced retinopathy. The authors concluded that monitoring HCQ blood levels can aid in identifying high-risk patients for retinopathy and facilitate dose adjustments.36
Nevertheless, a recommendation in the Clinical Practice Guideline on Systemic Lupus Erythematosus of Spanish National Health System could shed light on this question by suggesting monitoring disease activity, organ damage, comorbidities (including the presence of vascular risk factors), and possible toxicity of drug treatment. For this purpose, clinical interview, physical examination, blood pressure measurement, as well as basic analytical determinations that will include complete blood count, biochemical profile with renal function analysis, urinalysis, complement, and determination of anti-ds DNA antibodies can be used.37 Although these tests are suggested to monitor SLE patients in general, would also cover the rare eventuality of a hematologic or hepatic adverse effect of HCQ.
Level of evidence 4
Recommendation (expert consensus):
- 7.1.
No specific plasma determinations are considered necessary for toxicity monitoring. Given the absence of sufficient evidence at present, the use of HCQ plasma levels for toxicity monitoring or dose adjustment is not recommended in daily clinical practice.
Grade of recommendation D
Question 8. What is the recommended strategy for evaluating adherence to HCQ treatment in patients with SLE?
Evidence for the recommendation:
The evidence highlights the crucial role of treatment adherence in achieving effective outcomes for SLE patients.38 Non-adherence rates in SLE patients range between 10% and 50%.31
Two studies conducted by Costedoat-Chalumeau et al. measured HCQ adherence in SLE patients through drug analysis in blood and patient questionnaires.39,40 However, self-report questionnaires showed a weak correlation with HCQ concentration in blood, and a combination of both methods was recommended.41 Other studies used pharmacy refill records, pill counts, physician ratings, and the Haynes–Sackett test.38,42–44 Although total blood levels are preferred for measuring adherence, HCQ levels can also be monitored regularly in blood in SLE patients to ensure optimal dosing and adherence.45
A retrospective longitudinal study in South Korea evaluated HCQ adherence using the medication possession ratio (MPR), and non-adherence was defined as an MPR <0.8.46 Similarly, Liu et al. also used MPR to measure adherence.47
As for the pediatric population, a study evaluated a web-based education program with and without social media intervention and found that the combined intervention improved medication adherence in adolescents and young adults with SLE.48
Level of evidence 2+
Recommendation:
- 8.1.
It is recommended to focus on the clinical visit, asking the patient about adherence and verifying it through electronic prescription as most feasible strategy to monitoring adherence.
Grade of recommendation C
DiscussionThis consensus document addresses the variability in clinical practice regarding the use of HCQ in the treatment of SLE. It aims to provide practical evidence-based recommendations on the use of HCQ in the clinical management of SLE.
The optimal starting dose required continues to be a matter of contention.4 Evidence suggests a low risk of toxicity for doses equal to or under 5mg/kg/day (based on actual body weight). However, there is a suggestion of a higher dose of 6.5/mg/kg/day (based on ideal body weight), but it is not entirely known whether the risk–benefit trade off, including the prevention of certain comorbidities, will be the same in both cases.5 Likewise, the scientific committee highlighted, that for the pediatric population, HCQ treatment should be applied to all patients with SLE at a dose of 5mg/kg/day based on the new recommendations.
Cumulative exposure and potential HCQ-induced toxicity are the main reason to consider reducing HCQ during the course of the disease. Generally, it is not recommended to discontinue or gradually reduce the dose of HCQ, but in patients with a long evolution of the disease, the risk–benefit should be evaluated. The 2019 update of the EULAR recommendations highlights the efficacy of long-term use of HCQ in sustaining remission, suggesting reduction or discontinuation only in specific cases, and only with strong clinical justification.5 Continuing HCQ treatment in SLE patients is recommended not only to prevent relapse but also to achieve reduced disease activity, minimize flares, enhance survival rates, and lower the risk of cardiovascular complications. Additionally, long-term HCQ use has been associated with reduced infection rates and fewer thrombotic events in SLE patients.49
In this context, the scientific committee highlights the lack of clear evidence defining prolonged remission but suggests considering it if the absence of symptoms lasts for more than one year. In such situation, a risk-benefit evaluation may support dose reduction. Discontinuing treatment is not recommended except in cases of toxicity, particularly in the first year of SLE evolution where the risk of flare-up is higher upon HCQ discontinuation.
Although less evidence exists to this regard for pediatric patients, similar recommendations may be issued for children.
Adverse effect of HCQ, primarily retinal toxicity, can lead to irreversible retinal damage, but modern screening tests allow an early detection before any impact on visual function. Retinal toxicity is dose dependent, and the risk increases significantly with doses above 5mg/kg per day and with cumulative exposure.19,24,25 Regular ophthalmic monitoring is widely recommended,7,21,23,37 usually starting after 5 years of HCQ treatment and carried out annually.
The experts reached a consensus that there is currently no conclusive evidence to support the need for an ophthalmologic assessment before starting HCQ treatment. However, most published recommendations concur on the importance of conducting a baseline ophthalmological evaluation to rule out pathologies like macular degeneration or macular dystrophies that could contraindicate HCQ use and to establish a reference point for subsequent assessments and records any pre-existing macular abnormalities which might otherwise cause confusion and potentially lead to unnecessary discontinuation of HCQ.
The recommendations of medical societies include the performance SD-OCT and automated visual fields.7,21 The scientific committee's discussion regarding the type of tests to be performed for monitoring retinal toxicity SD-OCT should be mandatory at follow-up visits. In cases where OCT results are doubtful or pathological, a functional test, mainly visual fields should be carried out. Other complementary tests, such as Fundus Autofluorescence and multifocal electroretinogram (mfERG) are at the discretion of ophthalmologists.
When toxicity is diagnosed, a consensus decision with the specialist in charge of the patient is recommended instead of direct withdrawal. In cases of very early signs of toxicity, maintaining HCQ at a lower dose may be more beneficial than suspension.
The scientific committee emphasized the need for a referral ophthalmologist with special interest and experience in HCQ retinal toxicity to carry out annual check-ups and liaise with the prescribers.
In addition to retinal toxicity, the risk of cardiac events related to HCQ has also been documented.26,27 However, there is a lack of randomized controlled trials (RCTs) and large observational studies to confirm these findings. Experts’ remarks that there are two scenarios: cardiomyopathy and rhythm disturbances. With respect to cardiomyopathy, nothing needs to be done at baseline, however for cardiac screening for prolongation of the QT interval a baseline electrocardiogram is recommended in patients receiving HCQ.
Lack of adherence is a significant issue in routine clinical practice, especially among patients with chronic disease like SLE. While blood level measurement may be optimal for verifying treatment adherence, the lack of standardization and variability in methods, as well as the access limitations to this technique for a large number of centers make difficult to use it in routine clinical practice. The scientific committee mentioned some strategies that should be considered to enhance adherence, such as reducing the number of pills required per day, encouraging communication, recommending pillboxes, setting reminders, and providing education on the significance of adherence.
There are some limitations of this consensus document worth mentioning. Firstly, the lack of high-quality studies supporting more substantial recommendations presents a challenge, underscoring the need for further rigorous research in the field. Moreover, the composition of the evaluation committee, with only one ophthalmologist and the absence of a cardiologist, accentuates potential biases in the discussion regarding retinal toxicity and cardiac events associated with HCQ. Despite these limitations, this consensus document serves as a valuable reference for healthcare professionals.
In conclusion, this consensus aims to close the gap regarding the treatment of patients with SLE by providing practical and valuable recommendations for health professionals involved in the care of the disease.
Authors’ contributionsJCA participated in the conception and design of the study and all authors participated in the drafting the article and revised it. All authors approved the final version of the manuscript.
FundingThis study was founded by Gebro Pharma S.A. and the methodology of this consensus has been endorsed by the Spanish Society of Pediatric Rheumatology (SERPE) and the Spanish Society of Ophthalmology (SEO).
Conflict of interestThe authors declare the following potential conflicts of interest: IRF received fees for participating in the scientific committee of the consensus from Gebro Pharma. TCS received fees for participating in the scientific committee of the consensus from Gebro Pharma and participating in conferences and advisory meetings for GSK, Otsuka, Astra Zeneca, Rubió and Gebro Pharma. Declares has received an international and national scholarship from GSK and Astra Zeneca. JPR received fees for participating in the scientific committee of the consensus from Gebro Pharma and declares has received research funding from Astra Zeneca, GSK International, Pfizer International. He has agreements to participate in conferences or received fees as a speaker from Lilly, Pfizer, Astra Zeneca. He has participated in the advisory committee from Lilly, GSK, Astra Zeneca, MSD and Gebro Pharma. MG received fees for participating in the scientific committee of the consensus from Gebro Pharma. Has participated in scientific advisory for GSK, Astra Zeneca, Otsuka and received funding for scientific presentations from GSK, Astra Zeneca, Otsuka, Roche and Abbvie. Attendance to congresses for Abbvie, Lilly, Astra Zeneca, Otsuka. ED received fees for participating in the scientific committee of the consensus from Gebro Pharma. AFN received fees for participating in the scientific committee of the consensus from Gebro Pharma, Astra Zeneca. Attendance to congresses from Lilly. Has participated in scientific advisory for GSK, Astra Zeneca, Otsuka and received funding for scientific presentations from GSK. JRI received fees for participating in the scientific committee of the consensus from Gebro Pharma. ICP received fees for participating in the scientific committee of the consensus from Gebro Pharma. JA received fees for participating in the scientific committee of the consensus from Gebro Pharma. JCA received fees for participating in the scientific committee of the consensus from Gebro Pharma.
The authors would like to acknowledge Gebro Pharma for their generous support and funding. Additionally, they express gratitude to GOC Health Consulting for their methodological and medical writing support throughout the project's development.