Chikungunya (CHIKV), is an endemic RNA virus in some regions of Asia and Africa. In Colombia in 2014, its spread started explosively and quickly. The presentation of CHIKV is a febrile condition, with musculoskeletal symptoms, which can progress to erosive arthropathy and polyarticular deformity. The purpose of this study is to evaluate symptomatic and serological behaviour in patients suffering from CHIKV infection in Neiva, Huila who attend the Rheumatology clinic, and to describe the comorbidities associated with the chronic phase of the disease.
MethodsAn observational, longitudinal and retrospective analysis of data collected in 410 patients afflicted with the CHIKV virus, with symptoms lasting more than 3 months, who persisted with musculoskeletal and joint symptoms. The patients were classified according to their commitment in post-viral arthralgias, polyarthritis post viral, Rheumatoid Arthritis (RA) post CHIKV, Spondyloarthritis postCHIKV, and soft tissue rheumatism. The statistical analysis was performed using SPSS software (version 24). A descriptive analysis was carried out to evaluate quantitative variables such as the mean (standard deviation), and categorical variables such as frequency (%). The categorical variables were compared using the Chi-square equation. As a statistical significance, a p less than .05 was considered.
ResultsOf the 410 patients, 89.23% were women, with polyarticular involvement in 92.26% of the cases. Of the patients, 49.83% had osteoarthritis. At the time of the evaluation in the Rheumatology clinic, 46.3% of the cases presented persistent non-inflammatory arthralgias, and 53.7% of the patients underwent arthritis on physical examination, of which, remarkably, 20.3% met the criteria for rheumatoid arthritis postCHIKV.
ConclusionsThe development of musculoskeletal symptoms after CHIKV infection is a very serious public health problem, with persistent complications and long-term morbidity risk in real life. The presence of net postviral arthritis is noteworthy, however the development of postCHIKV rheumatoid arthritis usually requires more advanced pharmacological measures, including, in some cases, transition to biological therapy. The presence of symptoms of venous insufficiency in the lower limbs that developed with CHIKV infection was an incidental finding that requires a more precise study.
Chikungunya (CHIKV) es un virus RNA endémico en algunas regiones de Asia y África. En Colombia en el año 2014 inicia su dispersión de manera explosiva y rápida. La presentación del CHIKV es una condición febril con síntomas musculoesqueléticos que pueden progresar a artropatía erosiva y deformidad poliarticular. El propósito de este estudio es evaluar en pacientes aquejados por la infección por CHIKV en Neiva, Huila que acuden a la consulta de Reumatología, el comportamiento sintomático y serológico, y describir comorbilidades asociadas a la fase crónica de la enfermedad.
MétodosSe realizó un análisis observacional, longitudinal y retrospectivo, de datos recolectados en 410 pacientes aquejados por el virus CHIKV con síntomas de más de tres meses de duración que persistían con afecciones musculoesquelíticas y articulares. Los pacientes fueron clasificados según su compromiso en: artralgias postvirales, poliartritis postviral, artritis reumatoide (AR) postCHIKV, espondiloartritis postCHIKV y reumatismo de tejidos blandos. El análisis estadístico fue realizado usando SPSS software (versión 24). Se llevó a cabo análisis descriptivo para evaluar variables cuantitativas como la media (desviación estándar) y variables categóricas como la frecuencia (%). Las variables categóricas fueron comparadas usando la ecuación X2. Como significancia estadística se consideró una p<0,05.
ResultadosDe los 410 pacientes, 89,23% fueron mujeres, con compromiso poliarticular en 92,26% de los casos. El 49,83% de los pacientes presentaban de base osteoartritis. Al momento de la evaluación en la consulta de Reumatología, 46,3% de los pacientes cursaron con artralgias no inflamatorias persistentes y 53,7% de los casos presentaban artritis al examen físico, de los cuales, de manera remarcable 20,3% cumplieron criterios para AR postCHIKV.
ConclusionesEl desarrollo de síntomas musculoesquelíticos luego de la infección por CHIKV es un problema de salud pública bastante serio, con complicaciones persistentes y riesgo de morbilidad a largo plazo en la vida real. La presencia de artritis postviral neta es de resaltar, sin embargo, el desarrollo de AR postCHIKV requiere usualmente medidas de índole farmacológico más avanzadas, incluso, en algunos casos es necesario referir a los pacientes a terapia biológica. La presencia de síntomas de insuficiencia venosa en miembros inferiores desarrollados con la infección por CHIKV, fue un hallazgo incidental que requiere de un estudio más preciso.
Chikungunya virus (CHIKV), a RNA-type arbovirus of the Togaviridae family and Alfavirus genus, is transmitted by Aedes aegypti and Aedes albopictus and it is endemic in regions of Africa and Asia, especially in India.1–3 It has been known since 1952 and its first outbreak in Tanzania, and it has interepidemic periods that last from four to 30 years. In 2004 it was distributed worldwide, after the outbreak in the Indian Ocean and India.4–7 It reached the continent of America in 2013 in Saint Martin,8–10 and having established in Colombia, in 2014 there was a first imported case in the Dominican Republic, and it was confirmed on 19 July 2014 in Palmira, Valle, as well as the first indigenous case on 11 September 2014, in the district of San Joaquín, Mahates, Bolívar. Classically CHIKV presents with the symptoms of fever, associated with musculoskeletal symptoms that resolve in 87.9% of cases, although they may leave persistent chronic inflammatory sequelae in 5.9% of cases.11 It may also present as erosive arthropathy and deforming polyarthritis in 5.6% of cases (associated with positive anticitrulline antibodies [AntiCCP] 12). The studies of the osteomuscular symptoms associated with CHIKV vary widely, with ranges from 14.4% to 87.2%,13–15 with more recent estimations based on data from India and La Reunión, France, which indicate that approximately 47.57% of patients may have persistent symptoms at 20 months.14 More aggressive and persistent symptoms have been described in Latin America and Colombia above all, as shown in the Sucre cohort (39 cases, 89.7% at 15 months), Venadillo–Tolima (44.3%), and La Virginia—Risaralda (53.7%. Nevertheless, only from 1.4% to 20% are referred to Rheumatology for study and monitoring.16–18 The aim of this observational, longitudinal and prospective study is to characterise the symptoms of patients who have suffered chronic infection by CHIKV in the Rheumatology Department of a health centre in Neiva, Huila, who visited after referral by primary health centres, defining their serological behaviour as well as the factors leading to poor prognosis and a persistent limiting pathology. This is the first real-life study in our environment, in patients who were evaluated in a supraspecialised surgery to produce a complete evaluation.
Materials and methods433 patients were recruited, and financed by the Laboratorio de Investigación Humana (LIH) of Manuela Beltrán University, they were tested to confirm the presence of IgG antibody against Chikungunya (KIT ELISA MyBiosource, Inc. San Diego, CA). 23 patients who were serologically negative and/or who were positive but lacked complete data for analysis were excluded, leaving 410 patients. Patients were clinically evaluated by complete detailed physical examination and questioned while keeping the previous predictions of other cohort studies in mind.16–18 These predictions included the predominance of women aged over 40 years with previous joint pathologies, menopausal or not, a history of calcium supplementation, smoking and a family history of rheumatic pathology. The behaviour of the virus was evaluated, defining single joint symptoms, oligoarticular symptoms (two to four joints), and polyarticular symptoms (more than four joints), the date symptoms commenced, previous venous symptoms, a history of cardiovascular, joint or inflammatory comorbidities, current or previous smoking, family history of rheumatic pathologies, calcium consumption at the time of the evaluation, treatments prior to evaluation by Rheumatology (conventional treatments as well as natural medicine, which is widely used in our context for multiple health problems), and the presence of arthritis in clinical examination by an expert rheumatologist. Diagnoses of musculoskeletal pathologies are generally clinical, although ACR/EULAR 2010 classification criteria were used in patients with persistent polyarthralgias who fulfilled the criteria for Rheumatoid Arthritis (RA), as well as ASAS criteria for peripheral and axial spondyloarthritis (EspP and EspAx), ACR/EULAR criteria for systemic lupus erythematosus (SLE), BCDJ criteria for Behcet’s disease and ACR 2010 fibromyalgia diagnostic criteria. The patients with arthralgia due to the viral process but without signs of inflammation were classified as post-viral arthralgia, and those patients with inflammatory polyarticular progression who did not fulfil the criteria for classification as RA or another specific type of arthritis following the above-mentioned classification criteria were categorised as post-viral polyarthritis, as well as the patients with soft tissue pain, local swelling or the presence of any type of tenosynovitis, fasciitis, bursitis and/or localised non-inflammatory pain were classified as soft tissue rheumatism (including patients with fibromyalgia). Lastly, complementary paraclinical immunological tests were requested (according to evaluation of the relevance of this for the case), acute phase reagents in patients with clinically evident arthritis, for the prognostic prediction of the case, as well as variables such as thyroid studies, screening for osteoporosis and the measurement of 25 (OH) Vitamin D. All data were compiled, tabulated and statistically analysed using version 24.0 of SPSS, with descriptive analysis of each one of the variables, as well as uni- and bivariant analysis to determine strength of association using X2.
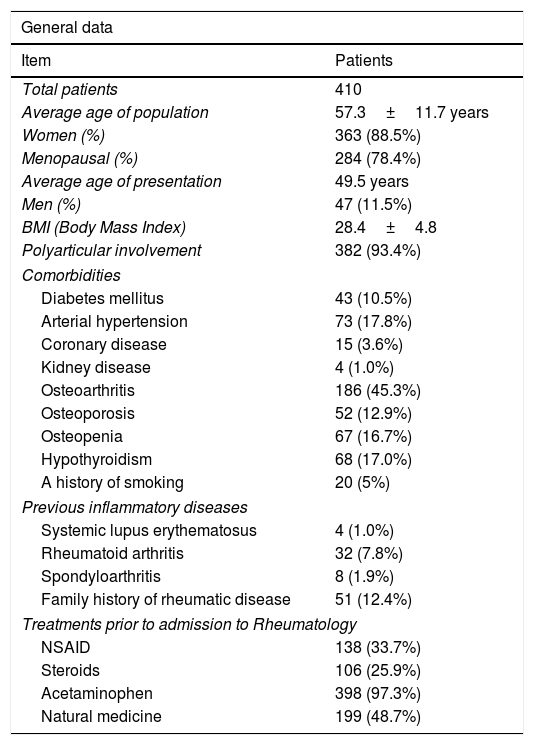
ResultsAt the outbreak of the epidemic of the virus in the Huila region it was decided to start gathering Rheumatology treatment data on patients who initially fulfilled the epidemiological criteria for CHIKV infection, using INS 2015 diagnostic criteria for clinically confirmed cases: fever above 38°C, severe arthralgia or arthritis with an acute onset, and multiform erythema or symptoms that could not be explained by other medical conditions, patients who lived in or had visited a town where there was evidence of circulating CHIKV virus, and who also fulfilled the criteria for chronic infection by CHIKV virus established by PAHO 2015,19 in patients who visited our Department from March 2015 to date (the cut-off point for publication was December 2019, patients with long-term involvement are still being seen for the first time in Rheumatology, given aspects of the local health system and the opportunity for specialised treatment). Of the total number of 410 patients who were recruited and accepted taking part in the follow-up, 363 were women (88.5%, of whom 78.4% were in a stable postmenopausal state) and 47 were men (11.5%), with an average age of 57.3±11.7 years old, predominantly with a commencement of the symptoms from December 2014 to February 2015 (69% of the cases), and predominantly polyarticular involvement (93.4% of cases). Table 1 shows pre-existing comorbidities in the patients, showing that some already had inflammatory pathology with re-exacerbation of the symptoms (7.8% with RA, 1.9% with spondyloarthritis and 1.0% with SLE). It should be underlined that 45.3% of the patients had clinically evident osteoarthritis (compatible with the age group older than 40 years, with greater persistence of the disease), previous use of oral or IM steroids (25.9% of cases), and a strikingly widespread use of natural therapy (48.7% of cases). 78.4% of cases were women after the menopause, with an average age at commencement of 49.5 years. The body mass index (BMI) of the whole cohort was 28.4±4.8kg/m2 SC.
Patient characteristics.
| General data | |
|---|---|
| Item | Patients |
| Total patients | 410 |
| Average age of population | 57.3±11.7 years |
| Women (%) | 363 (88.5%) |
| Menopausal (%) | 284 (78.4%) |
| Average age of presentation | 49.5 years |
| Men (%) | 47 (11.5%) |
| BMI (Body Mass Index) | 28.4±4.8 |
| Polyarticular involvement | 382 (93.4%) |
| Comorbidities | |
| Diabetes mellitus | 43 (10.5%) |
| Arterial hypertension | 73 (17.8%) |
| Coronary disease | 15 (3.6%) |
| Kidney disease | 4 (1.0%) |
| Osteoarthritis | 186 (45.3%) |
| Osteoporosis | 52 (12.9%) |
| Osteopenia | 67 (16.7%) |
| Hypothyroidism | 68 (17.0%) |
| A history of smoking | 20 (5%) |
| Previous inflammatory diseases | |
| Systemic lupus erythematosus | 4 (1.0%) |
| Rheumatoid arthritis | 32 (7.8%) |
| Spondyloarthritis | 8 (1.9%) |
| Family history of rheumatic disease | 51 (12.4%) |
| Treatments prior to admission to Rheumatology | |
| NSAID | 138 (33.7%) |
| Steroids | 106 (25.9%) |
| Acetaminophen | 398 (97.3%) |
| Natural medicine | 199 (48.7%) |
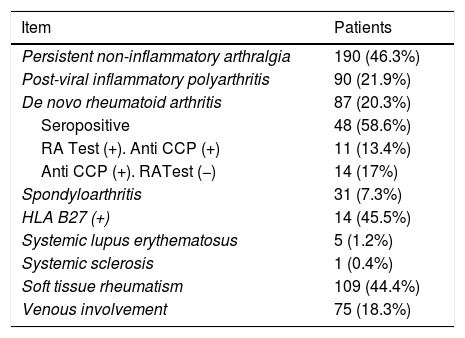
Table 2 shows patient behaviour, with the initial evaluation by Rheumatology that 46.3% of the patients fulfilled the criteria for persistent non-inflammatory arthralgia, while 53.7% of the patients were found to have arthritis in the physical examination. After the initial paraclinical tests (measurement of autoantibodies, acute phase reagents and case confirmation by CHIKV IgG measurement), as well as clinical assessment by an expert in Rheumatology, the said patients were sub-classified as follows: 21.9% (90 patients) had net post-viral inflammatory polyarthritis, 20.3% (87 patients) were diagnosed de novo post-CHIKV RA (58.6% of whom were seropositive and 11 patients (13.4%) were doubly seropositive for the RA test/AntiCCP positive, and remarkably 14 patients (17%) were AntiCCP positive with a negative RA test); 7.3% (31 patients) were diagnosed with axial and/or peripheral spondyloarthritis (15 patients with peripheral involvement, 11 patients with axial involvement and five patients with development of psoriatic arthropathy), with the presence of HLA-B27 positive in 46.7%, 45.5% and 0% of cases, respectively; 1.2% (five patients) developed criteria for SLE, all of them with joint involvement, with no involvement of soft organs and controlled with the use of chloroquine+azathioprine; and 0.4% (one patient) developed systemic sclerosis with limited cutaneous involvement and pulmonary involvement that required treatment with cyclophosphamide. A simple lineal regression model was prepared in SPSS for statistical analysis, calculating the strength of association with a 95% confidence interval. In this it was possible to identify the following as risk factors for the development of post-CHIKV RA: the presence of positive anticitrulline antibodies (P=.000), positive PCR (P=.000), raised VSG (P=.002), as well as pre-existing osteoarthrosis (P=.001).
Diagnostic classification at admission.
| Item | Patients |
|---|---|
| Persistent non-inflammatory arthralgia | 190 (46.3%) |
| Post-viral inflammatory polyarthritis | 90 (21.9%) |
| De novo rheumatoid arthritis | 87 (20.3%) |
| Seropositive | 48 (58.6%) |
| RA Test (+). Anti CCP (+) | 11 (13.4%) |
| Anti CCP (+). RATest (−) | 14 (17%) |
| Spondyloarthritis | 31 (7.3%) |
| HLA B27 (+) | 14 (45.5%) |
| Systemic lupus erythematosus | 5 (1.2%) |
| Systemic sclerosis | 1 (0.4%) |
| Soft tissue rheumatism | 109 (44.4%) |
| Venous involvement | 75 (18.3%) |
The pharmacological interventions in each group of patients also different, depending on their diagnosis. Thus 75.5% of the patients who met the criteria for post-viral polyarthritis were treated with chloroquine with an acceptable clinical response (68 patients), while 24.5% (22 patients) required FARME monotherapy with methotrexate; unlike the patients with RA, who required initial monotherapy with methotrexate in 65.1% of cases (56 patients), combined FARME therapy with methotrexate/leflunomide in 10.5% of cases (nine patients), and, interestingly, six patients required a switch to biological therapy (three patients with de novo post-CHIKV RA and three patients with pre-existing RA whose conventional FARME treatment failed after the infection).
DiscussionIn December 2013 the PAHO issued an epidemiological alert after the CHIKV virus was detected for the first time on the American Continent,20 meaning that the virus “had arrived to stay”. Unfortunately, and in spite of the warnings given, the prevention mechanism is insufficient in Andean region countries such as Colombia. This gave rise to a greater public health problem, particularly in warm tropical areas where other conditions already exist, such as Dengue fever.8,21 In Colombia, since the first native case of CHIKV was recorded in the San Mahates, Bolívar region in September 2014, the infection became established very quickly, and more than 200,000 confirmed cases were recorded in the first months of 2015. The presence of osteoarticular and musculoskeletal complications has increased, leading to more visits due to persistent pain; likewise, in many cases the development of permanent inflammatory pathology that is limiting and even in some advanced cases a consequence (referring to other cohorts such as chronic post-CHIKV — RIC inflammatory rheumatism) required advanced intervention in the Rheumatology Department. Another finding was that 20.3% of patients with persistent symptoms developed RA with clinical behaviour very similar to conventional RA, and with very similar data to those previously found in La Reunion, France.22,23 After observation we decided on the need for aggressive treatment of these cases, and even, in some of these patients, biological therapy was required, keeping in mind the fact that the initial presence of anticitrulline antibodies (with no Rheumatoid factor), positive PCR, raised VSG and/or a previous history of osteoarthrosis, have functioned as risk factors in facilitating the development of chronic inflammatory pathology. Likewise, the development of other pathologies such as axial or peripheral spondyloarthitis in previously asymptomatic patients (with association to HLA-B27 in 45.5% of cases), as well as the isolated appearance of five cases of systemic lupus erythematosus (which was not present beforehand) and one case of systemic sclerosis should also be noted, as observations in our cohort.
Strikingly, and in agreement with other cohorts that evaluate the cases of patients with chronic conditions, pre-existing situations have been defined as inflammatory process triggers and perpetuators, aided to a large degree by the population involved, which usually has associated comorbidities, are older than 45 years, and the severity and duration of the initial inflammatory phase.24–26 Although in some cohorts being of female sex was not significantly associated (as in our case) with being a predisposing factor,27–29 in our cohort the majority of cases were postmenopausal women with previous joint symptoms, chiefly osteoarthritis, with no calcium intake and a BMI within the ranges of overweight or obesity. Additionally, the presence of soft tissue rheumatism (also referred to as post-CHIKV musculoskeletal disorders),30 in comparison with cohorts such as the one in India, show a higher percentage. This makes it possible to suggest a greater susceptibility to symptoms in postmenopausal women who are not taking calcium supplements and who have a tendency to become overweight; however, the suspicion also increases that there may be muscle fibre involvement promoted directly by the virus (which has been examined more in the acute phase, although without any clear certainty in cases of chronic inflammation), given the action of IL-6, MCP-I, IL-8, MIF, GM-CSF, and C3 activation. Two very interesting items of data should be remarked on as particularities found in patient observation: (1) the development of soft tissue rheumatism was found in 44.4% of the patients, which is far higher than the corresponding figure in the Indian cohort (27.7%); of these patients, 71.4% are postmenopausal women, 26.6% have Vitamin D deficiency (<30ng/dL), and 19.5% have autoimmune thyroid pathology as the cause of their painful symptoms; (2) the development of lower limb static venous pathology or the worsening of their basal condition was found in 18.3% of the patients; this situation had not been found in previous studies or cohorts, so that a more exact study is required, given that there are no previous causes for the said symptom, and that when associated with the other symptoms it too becomes a reason for consultation and a limiting factor for patients.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank Manuela Beltrán University for its support of the Health Research Laboratory.
Please cite this article as: Segura-Charry JS, Parada-Martinez MA, Segura-Puello HR, Muñoz-Forero DM, Nieto-Mosquera DL, Villamil-Ballesteros AC, et al. Desórdenes musculoesqueléticos crónicos por virus Chikungunya: experiencia real en la consulta de reumatología en Neiva, Huila. Reumatol Clin. 2021;17:456–460.