Transverse myelitis is a rare focal inflammation of the spinal cord. Multiple etiologies have been identified including autoimmune diseases, mainly systemic lupus erythematosus and Sjögren’ syndrome. It can occur in an acute or subacute clinical onset, with the acute presentation having a worse prognosis. An early diagnosis and intensive treatment are important features recommended in these patients.
We present three cases with transverse myelitis associated with autoimmune diseases. We discuss different clinical manifestations, association with autoantobodies, radiologic findings, and therapeutic and prognostic issues.
La mielitis transversa es una inflamación focal poco frecuente de la médula espinal. Su etiología es múltiple y entre ellas se encuentran las enfermedades autoinmunes, incluyendo principalmente el lupus eritematoso sistémico y el síndrome de Sjögren. Su presentación clínica puede ser de forma aguda o subaguda, con peor pronóstico en la mielitis transversa aguda. Un diagnóstico precoz y tratamiento intensivo desde el inicio es de gran importancia en la evolución de este tipo de pacientes.
Presentamos 3 casos con mielitis transversa asociados a enfermedades autoinmunes y discutimos sus distintas manifestaciones clínicas, la asociación a autoanticuerpos, las imágenes radiológicas, el tratamiento y el pronóstico.
Transverse myelitis (TM) is an inflammatory disease that causes a focal lesion of the spinal cord. It can affect anyone regardless of familial predisposition, ethnicity, gender or age, and has an incidence of 1.34–4.36 million persons affected per year, with 2 peaks of incidence: between 10 and 19 and between 30 and 39 years of age.1,2 Its etiology is multiple and among it are autoimmune diseases, showing a greater association with systemic lupus erythematosus (SLE) and Sjögren's syndrome (SS) in 1–2 and 1%, respectively.3,4 Its clinical characteristics are characterized by acute or subacute, sensory and autonomic level involvement at the level of the spinal motor system lesion.5 We report 3 cases of TM-associated autoimmune diseases diagnosed in our hospital in the last 3 years.
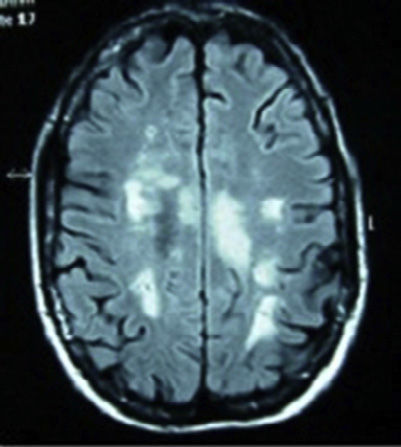
Clinical casesCase 1: 34-year-old Caucasian female, with a history of optic neuritis of the right eye 8 years ago. After admission due to abdominal discomfort, vomiting and fever, she presented 48h of ascending numbness and weakness of the lower limbs, urine and stool retention and decreased visual acuity of the left eye. During the interrogation in search of connective tissue diseases she referred xerostomia, xerophthalmia, vaginal and skin dryness. Neurological examination showed paraparesis with muscle balance of 1/5 in the left lower limb, 0/5 in the right lower limb and 2/5 in the right upper limb, diffuse hyperreflexia, extensor cutaneoplantar reflex, hypoesthesia for thermal sensitivity and in the painful D4-D5 dermatome, predominantly right deep sensitivity deficit in the left leg. Bilateral lower Schirmer test 5mm. Blood tests showed ESR 48mm/h, C-reactive protein 2.74mg/dl, antinuclear antibodies (ANA) speckled pattern +1/320, positive anti-Ro/SSA and aquaporin 4 antibodies and negative antiphospholipid antibodies. Vitamin B 12, folic acid, serology (syphilis, Brucella, Borrelia, HIV, HCV, HBV), Bence Jones protein, cryoglobulin, copper, serum levels of angiotensin converting enzyme, homocysteine and coagulation were normal. Magnetic resonance imaging (MRI) of cervical and thoracic spine: intramedullar lesion from C5 to D2. Cranial MRI (Fig. 1): presence of multiple lesions in the white matter on T2 and FLAIR. Evoked potentials: P100 response bilaterally elongated, being higher on the right side. Diagnosed with SS, treatment with boluses of methylprednisolone was initiated intravenously followed by oral prednisone at 50mg/day and azathioprine, achieving a progressive increase in visual acuity, although still with a temporal peripheral superior scotoma in the right eye. There was recovery of muscle balance and control of sphincters, walking without support at 15 months and without a new episode in the past 2 years.
Cranial MRI in T2 and FLAIR sequences, with presence of multiple lesions in the white matter at the subcortical, periventricular, periauricular and right corona radiata levels. The largest lesion extends from the posterior limb of the right internal capsule to the right mesencephalic peduncle.
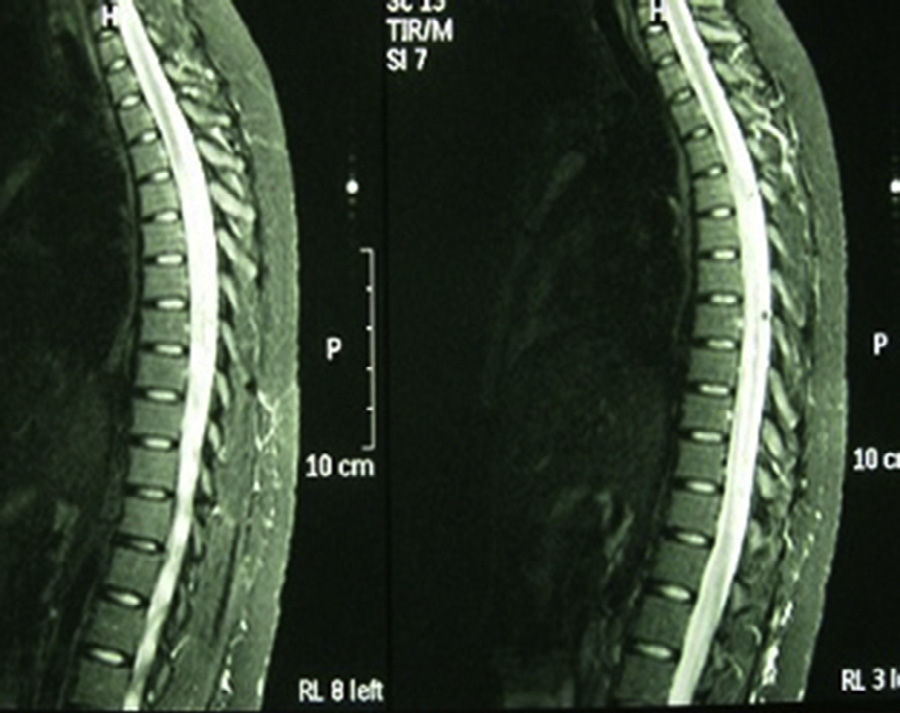
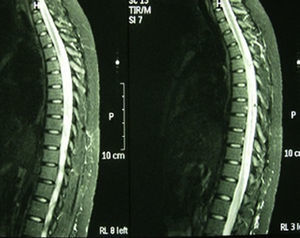
Case 2: 63-years-old female Caucasian, with a history of autoimmune hypothyroidism, hypercholesterolemia and 3 previous episodes of cervical myelitis of inflammatory origin, with a good response to bolus methylprednisolone. She came to the hospital for neck pain accompanied by decreased strength in the left upper and lower extremities, loss of sensation and dysesthesia in the left forearm and hand, progressive decrease in visual acuity and urinary incontinence. Upon interrogation she referred xerophthalmia and xerostomia of 3 years of evolution. Examination showed muscle balance in the upper left extremity of 2/5 distal and 3/5 proximal, in the left lower limb 2/5 proximal and distal, and the right leg 4+/5 proximal. Cutaneoplantar bilateral extensor reflex. Normal eye fundus. Schirmer test positive. Laboratory: blood count, biochemistry, thyroid hormones, protein, vitamin B12 and folic acid, normal. ANA, neutrophil cytoplasmic antibodies and antiphospholipid antibody, negative. Aquaporin 4 antibody positive. Serology for HIV, hepatitis A virus, HBV, HCV, Brucella and Borrelia negative. Lumbar puncture showed cerebrospinal fluid with 8leukocytes/mm,3 glucose 50.3mg/dl, protein 65.2mg/dl, adenosine deaminase 4.8U. Cultures for fungi, mycobacteria, Gram stain and cryptococcus, negative. MRI of cervical spine: cervical spinal involvement from the odontoid process to C5. Evoked potentials: reproducible response in both eyes, but increased latency in the left eye and borderline on the right, compatible with optic neuritis. Biopsy of salivary glands: salivary gland lobes with 2 compact aggregates of more than 50 mature lymphocytes consistent with SS. Biweekly treatment with cyclophosphamide and azathioprine was started and lasted 3 months, with significant progressive functional recovery in muscle balance 5/5 after the first year of treatment and no new episode of myelitis in the past 3 years. MRI of the skull and spine does not show areas of active myelitis.
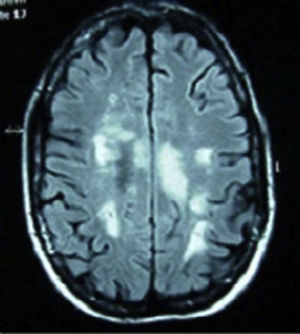
Case 3: 22-year-old Caucasian female, diagnosed with SLE at age 15 on the basis of polyarthritis, cutaneous and mucosal involvement, lymphopenia, positive ANA and anti-DNA at high titers. A renal biopsy due to persistent urinary abnormalities showed type IV lupus nephritis, and therapy with cyclophosphamide, prednisone and hydroxychloroquine was started. She came to the clinic with lumbar pain, dysuria, and asthenia, with gradual introduction of paresthesias in the right abdomen and lower limb paresis. Laboratory tests showed a WBC count of 30,000×910 (no left shift), erythrocyte sedimentation58mm/h, C-reactive protein6.41mg/dl and lactic acid 1.10mmol/l. Repeated analysis of blood and urine cultures were negative. Serology showed positive ANA 1/320, anti-DNA of 167U/ml, negative antibody antiphospholipid antibodies. MRI of the spine (Fig. 2) reported a large segment of myelophaty in the dorsal spinal cord. Cranial MRI changes observed in the left cerebellar hemisphere were a signal that could be related to lupus vasculitis, showing diffuse hyperintense signal in FLAIR and T2. No gadolinium was used upon patient request. After a lumbar puncture, a normotensive transparent liquid was obtained with 58mm3, 80% polymorphonuclear cells, 20% lymphocytes, glucose 127mg/dl, Protein1015mg/dl and lactate dehydrogenase 216U/l. Gram staining of cerebrospinal fluid showed no bacteria and fungal culture and CO2 were negative. She was treated with bolus methylprednisolone and 48h later she presented seizures which were resistant to treatment with valproic acid. She was interned in the intensive care unit, where treatment was initiated with phenytoin and midazolam for seizure control as well as tuberculosis treatment, cephalosporin and prophylactic acyclovir. After a feverish peak she showed an abrupt neurological deterioration, with Glasgow 8/15. Cranial computed tomography showed a massive and generalized cerebral edema with effacement of sulci and cisterns, not differentiating between white and gray matter. Contrast administration showed that intracranial circulation barely existed, with widespread signs of ischemia. Bradycardia and complex aberration triggered cardiorespiratory arrest.
DiscussionTM is a rare neurological impairment that can occur in autoimmune diseases, particularly in SLE and SS. In SLE it is more common in patients with antiphospholipid antibodies, or secondary antiphospholipid syndrome. This raises suspicion of its involvement in the pathophysiologic mechanism of spinal cord necrosis due to arterial thrombosis, in addition to the direct interaction between antibodies and spinal phospholipids.6 However, in our case of SLE, the many immunological studies conducted never showed antiphospholipid antibodies. Furthermore, biopsies and autopsy studies show inflammatory changes with perivascular infiltration of monocytes and lymphocytes. Thus, from this immunological point of view, various theories have been proposed. The first is the molecular mimicry between infectious antigens and common antigens with the spinal cord that produce immune response confusion. The second is that the overstimulation of T lymphocytes by microbial superantigens is capable of activating the immune system. Finally, a specific spinal lesion could be caused by the development of abnormal antibodies, like aquaporin-4 antibodies.7 These antibodies, also called NMO IgG are specific to NMO and are present in TM, with a sensitivity of 91% and a specificity approaching 100%. Titers decline with immunosuppressive therapy and remain decreased during remission. They can appear in extensive TM without optical myelitis, although are considered its precursors.8
It comes in the form of acute or subacute TM (over 4h and less than 4 weeks duration), or progressive myelopathy syndromes remission and relapse of chronic spinal cord involvement. Acute and subacute forms are associated with intense pain in the neck and interscapular region, followed by sensory and motor deficits below the level of spinal cord injury. Also subacute and chronic forms are most often associated with sensory symptoms, urinary incontinence and difficulty in walking which may progress to spastic paraplegia. The most commonly affected region is the cervical and upper thoracic spinal cord.9 In SLE, the predominant symptom is loss of strength in the lower limbs (70%), swelling of the affected area (47%), fever (21%), acute urinary retention (16%) and abdominal or lumbar (30%) pain. In SS, rapid onset myelitis have a sudden motor and sensory involvement, with cervical and interscapular pain, presenting a significant mortality rate. In the subacute form, it is associated with urinary disturbances, optic neuritis and eventually paraplegia.10
It may be the initial manifestation of SLE, presenting in the first 5 years from the diagnosis of the disease in 42%.3 Central nervous system affection in SS occurs in about 5%–8% of patients, constituting the first manifestation of the disease in half of the cases. Approximately one third of this involvement refers to myelopathy and this is the first manifestation of SS in 61% of these patients.11
Regarding diagnosis, MRI is the determining test. Studies show abnormality of signal with hyperintensity on T2, gadolinium enhancement and edema of the spinal cord. No specific images exist by etiology, although multiple small lesions seem indicative of SLE, while very extensive lesions and abnormalities at various levels are more indicative of vasculitis.12 On brain MRI in T2/FLAIR demyelinating lesions, with marked involvement can be found, as in our case 1. The study of cerebrospinal fluid may show inflammation of the spinal cord, with pleocytosis or elevated IgG values. In the basic immunological study we find antimyelin antibodies (which can predict the evolution of a clinically isolated syndrome to multiple sclerosis) and antiaquaporin 4 antibodies. Recurrence of TM has been associated with the presence of anti-Ro/SSA antibodies.13 However, it is precisely our case of SS with repeat episodes (case 2) which does not have anti-Ro/SSA or anti-La/SSB antibodies, basing the diagnosis on clinical and histological criteria.
Treatment is not standardized. The accepted therapeutic conduct is infusion of boluses of methylprednisolone and cyclophosphamide in the acute phase, followed by oral prednisone and cyclophosphamide in monthly boluses lasting 3–12 months. Other immunosuppressants, including azathioprine, cyclosporine and methotrexate, can also be considered, as well as plasmapheresis and, in resistant cases, IV immunoglobulin. Recent use in patients of rituximab after resistance to conventional therapy showed a lower relapse with progressive increase in functional capacity.14,15
Recovery, if it occurs, is usually observed at 8 weeks with a faster evolution at 3–6 months, and continues more slowly for up to 2 years. Approximately 50% have a full recovery, 29% partial and 21% do not improve or worsen, with sequela in gait disturbance, urinary and gastrointestinal symptoms, including death.10
ConclusionIn the differential diagnosis of myelopathy, the clinician must consider autoimmune diseases which, although not a common cause, may be the first manifestation of the disease and their importance increases with the recurrence of episodes and the association with optic neuropathy. Antiaquaporin 4 antibody levels are found in high numbers or exist in the first episode and are associated to recurrence.
The importance of early diagnosis and aggressive treatment from the onset of the disease is vital, both in the clinical process and its aftermath.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this research did not perform experiments on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients, and all patients included in the study have received sufficient information and gave written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained informed consent from patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflict of InterestThe authors have no disclosures to make.
Please cite this article as: Menor Almagro R, Ruiz Tudela MM, Girón Úbeda J, Cardiel Rios MH, Pérez Venegas JJ, García Guijo C. Mielitis transversa en síndrome de Sjögren y lupus eritematoso sistémico: presentación de 3 casos. Reumatol Clin. 2015;11:41–44.