To describe the methods of the Spanish Registry of patients with idiopathic inflammatory myopathy (IIM) (Myo-Spain), as well as its strengths and limitations. The main objective of the project is to analyse the evolution and clinical management of a cohort of patients with IIM.
MethodsObservational, longitudinal, ambispective and multicentre study of a cohort of patients with IIM seen in rheumatology units in Spain. All patients with a diagnosis of IMM will be included in the regular follow-up of the participating centres, regardless of age on initiation of the process. Incident cases will be all patients who at the beginning of the study have been diagnosed for less than 12 months and prevalent cases for more than 12 months. The registry will include data from the visit at baseline, one year and two years. Socio-demographic, clinical, analytical variables, complications, comorbidities, association with other rheumatic diseases, hospital admissions, mortality and treatments will be collected. In addition, indices, scales and questionnaires of activity, muscle involvement, damage, disability, and quality of life will be determined. The recruitment period will be 23 months. The purpose is to obtain a cohort of 400 patients with IMM.
ConclusionsMyo-Spain registry provides the opportunity to develop a cohort of incident and prevalent patients with IMM in Spain. Myo-Spain will be able to assess in detail the clinical characteristics of the disease at different times. The comprehensive information collected during the visits is expected to provide a broad source of data for future analysis.
Describir la metodología del Registro de pacientes con miopatía inflamatoria idiopática (MII) de España (Myo-Spain), así como sus fortalezas y limitaciones. El objetivo principal del proyecto es analizar la evolución y el manejo clínico de una cohorte de pacientes con MII.
Material y métodoEstudio observacional, longitudinal, ambispectivo y multicéntrico de una cohorte de pacientes con MII atendidos en servicios de Reumatología de España. Se incluirán todos los pacientes con diagnóstico de MII en seguimiento habitual por los centros participantes, sin tener en cuenta la edad de inicio del proceso. Los casos incidentes serán todos los pacientes que al inicio del estudio en cada centro estén diagnosticados desde hace menos de 12 meses y casos prevalentes desde hace más de 12 meses. Se construirá un registro en el que se incluirán los datos de la visita basal, del año y dos años. Se recogerán variables socio-demográficas, clínicas, analíticas, complicaciones, comorbilidad, asociación con otras enfermedades reumáticas, ingresos hospitalarios, mortalidad y tratamientos. Además, se determinarán índices, escalas y cuestionarios de actividad, afectación muscular, daño, discapacidad y calidad de vida. El periodo de reclutamiento será de 23 meses. El propósito es conseguir una cohorte de 400 pacientes con MII.
ConclusionesEl estudio Myo-Spain constituye la oportunidad para desarrollar una cohorte de pacientes incidentes y prevalentes con MII en España. Myo-Spain permitirá evaluar en detalle las características clínicas de la enfermedad en diferentes momentos. Se espera que la información exhaustiva recogida en las visitas suponga una amplia fuente de datos para futuros análisis.
Idiopathic inflammatory myopathies (IIM) form part of a heterogeneous group of diseases of autoimmune origin characterised by non-suppurative inflammation of the skeletal muscles, progressive muscle weakness and variable expressivity in different organs and systems.1 IIM are rare, with a mean incidence ranging from 1 to 19 cases/million inhabitants/year,2,3 and vary according to geographical area, methodology and classification criteria. A study on the estimated incidence in Spain between 1997–2004 showed an incidence rate of 8.9 new cases/million inhabitants/year (95% confidence interval [CI] 8.6–9.2), with a higher incidence of dermatomyositis (DM) than polymyositis (PM).4 The aetiology and predisposing factors for developing IIM are still not entirely clear. They are currently considered immune-mediated disorders, possibly triggered by environmental factors in genetically predisposed individuals.5 IIM presents with a wide variety of clinical manifestations; muscle weakness secondary to chronic inflammation of striated muscle is characteristic. Other organs affected include the skin, joints, lung, gastrointestinal tract, and heart. Complications include infections and cancers.6–8 Although the prognosis has improved substantially in recent decades, mortality has increased with respect to the general population.9 Furthermore, at least one third of patients are moderately or severely disabled, mainly due to pulmonary involvement, the development of calcinosis and muscle weakness.10
The identification of specific and associated antibodies, access to imaging techniques such as magnetic resonance imaging and ultrasound, improvements in the interpretation of muscle biopsy and the development of new classification criteria in recent decades have given us a better understanding of this group of diseases.11
However, the heterogeneity of their clinical manifestations, their evolution and prognosis, along with their low prevalence and incidence, make study of these diseases difficult. In Spain, prospective studies are limited, especially those that include all IIM subtypes. No information is available on disease activity, severity, disability, quality of life, treatment management or prognostic factors. In addition, it has not been studied whether the disease behaves differently when comparing patients who meet the new American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) classification criteria at diagnosis with those who meet them during follow-up, or those who never meet them.
Therefore, the Myo-Spain project (Spanish Registry of patients with idiopathic inflammatory myopathy (IIM)) was set up with the main objective of analysing the evolution and management of a cohort of patients with IIM attended in rheumatology departments in Spain. This paper describes the project’s methodology and potential strengths and limitations.
MethodologyGeneral designAn observational, longitudinal, ambispective and multicentre study. An initial follow-up of two years has been proposed, which is expected to be longer to achieve the project’s main objective (to study the evolution and management of IIM). The full study protocol is available at https://myospain.ser.es.
Reference populationPatients diagnosed with any type of IIM under routine follow-up by rheumatology services, regardless of age at onset.
Two groups of patients have been considered according to time of diagnosis. Incident cases will be all patients who at the start of the study in each centre have been diagnosed for less than 12 months and prevalent cases, all those diagnosed for more than 12 months.
Study population. Inclusion and exclusion criteria1. Inclusion criteria
- -
Patients with a diagnosis of IIM, according to the judgement of the attending physician, and under active follow-up. Types of IIM can be DM, PM, amyopathic DM, hypomyopathic DM, inclusion body myositis (IBM), immune-mediated necrotising myopathy (IMNM), juvenile myopathies, anti-synthetase syndrome, other overlapping syndromes with myositis and unclassifiable.
- -
No age group is excluded from the study. The questionnaires will be answered by the parents or guardians of non-mature minors.
2. Exclusion criteria
- -
Patients who, in the opinion of the investigator, have difficulties in attending the visits or completing the forms.
- -
Individuals diagnosed with myopathies of toxic or infectious cause or secondary to neuromuscular disease.
- -
When complete data required for the classification criteria, or a final medical diagnosis are not available.
All hospitals with specialist rheumatology care belonging to the Spanish Society of Rheumatology Systemic Autoimmune Diseases Working Group (GT EASSER) and interested members of the Spanish Society of Rheumatology (SER) were invited to participate. An online survey was conducted (June 2017) that obtained information on the experience and casuistry of IIM. A pre-selection was then made using an application form sent by interested centres (7 March to 10 April 2018), detailing recruitment capacity and experience in the management of IIM. The centres were selected over the month of June 2018. A total of 32 centres are eventually participating in the project (Table 1).
List of participating centres.
| Participating hospital | Autonomous community |
|---|---|
| Hospital Universitario de Badajoz | Extremadura |
| Hospital Universitario de la Santa Creu i Sant Pau | Catalonia |
| Complejo Asistencial Universitario de Salamanca | Castilla y León |
| Hospital Universitario 12 de Octubre | Community of Madrid |
| Hospital del Mar | Catalonia |
| Hospital General Universitario Gregorio Marañón | Comminity of Madrid |
| Hospital Universitario Puerta de Hierro | Community of Madrid |
| Hospital General Universitario de Alicante | Valencian Community |
| Hospital Universitario de Bellvitge | Catalonia |
| Hospital General Universitario de Elda | Valencian Community |
| Hospital Universitario Virgen del Rocío | Andalusia |
| Complejo Hospitalario Universitario A Coruña | Galicia |
| Hospital Universitario Ramón y Cajal | Community of Madrid |
| Hospital Universitario de la Princesa | Community of Madrid |
| Hospital Universitario de Basurto | Basque Country |
| Hospital Germans Trias i Pujol | Catalonia |
| Hospital General de Granollers | Catalonia |
| Hospital Universitario de Gran Canaria Doctor Negrín | Canary Islands |
| Hospital Universitario Infanta Sofía | Community of Madrid |
| Hospital Universitario la Paz | Community of Madrid |
| Hospital Universitario Fundación Jiménez Díaz | Community of Madrid |
| Hospital Universitario HM Sanchinarro | Community of Madrid |
| Complejo Hospitalario Universitario de Vigo | Galicia |
| Hospital Universitario Son Llàtzer | Balearic Islands |
| Hospital Universitario Virgen de la Arrixaca | Community of Murcia |
| Hospital Universitario de Canarias | Canary Islands |
| Hospital Universitario Donostia | Basque Country |
| Hospital Universitario Reina Sofía | Andalusia |
| Hospital Clínico Universitario de Valencia | Valencian Community |
| Complejo Hospitalario Universitario de Santiago | Galicia |
| Hospital Universitario Vall d’Hebron | Catalonia |
| Hospital Universitario Príncipe de Asturias | Community of Madrid |
The recruitment period is 23 months. The aim is to recruit all patients with a diagnosis of IIM who meet the inclusion criteria with none of the exclusion criteria. All patients must provide their informed consent prior to entering the study.
Patients are invited to participate consecutively at one of their regular visits to the rheumatologist. Patient access to rheumatology services for diagnosis and follow-up is as routine (emergency department, primary care and interconsultation with other specialties such as dermatology, pulmonology, or neurology). The study has been opened to collaborating researchers from other specialties such as neurology and internal medicine, although rheumatology will coordinate in each centre. The recruitment period began in June 2019.
The electronic Data Collection Form (DCF) allocates an identification code in the study to each of the participants, to maintain data confidentiality, in accordance with current legislation (EU Regulation 2016/679 of the European Parliament and of the Council of 27 April 2016 on Data Protection [RGPD]), as well as all applicable Spanish and European legislation on privacy and personal data protection. The study has been approved by the reference (Hospital La Paz, Madrid) clinical research ethics committee (CREC) and by each of the CEICs of the participating centres.
Data collectionAn electronic DCF specific to Myo-Spain has been created to which each participating centre has access. Clinical information is collected retrospectively (prevalent cases) from the clinical history, and prospectively (prevalent and incident cases), obtained from baseline and follow-up visits, after one and two years.
Variables and measurementsMultiple analyses with different outcome variables are necessary to meet the objectives of the project, and therefore no specific primary outcome variable has been defined.
Variables and measurements will be collected on the online DCF, both at baseline and at the follow-up visit, after one and two years.
- 1
General, inclusion and sociodemographic data
These variables include date of birth, sex, race, educational level, the patient’s IIM classification criteria, type of IIM, date of onset of IIM symptoms, data of diagnosis of IIM, and date of visit. In addition, the date and reason for loss to follow-up will be recorded at the follow-up visits of year 1 and year 2 for patients who drop out of the study.
- 2
Classification criteria
Variables relating to the classification criteria are recorded, such as the form of onset of IIM (acute [0–14 days from onset], subacute [>14 days to ≤2 months] or insidious [>2 months to years]), weakness in the limbs and in the flexor-extensor musculature of the neck, muscle pain on pressure or spontaneous muscle pain, elevated muscle enzyme levels, presence of typical DM rash, joint, digestive and systemic manifestations, muscle biopsy, electromyography and magnetic resonance imaging results.
- 3
Disease activity
This includes the Myositis Disease Activity Assessment (MYOACT) where the physician assesses disease activity over the last four weeks using a 10-cm visual analogue scale (VAS) in seven organs/systems (constitutional, cutaneous, skeletal, gastrointestinal, pulmonary, cardiac, and muscular). It also includes global extramuscular assessment and global MYOACT assessment (muscular and extramuscular) in the last four weeks on a 0−10-cm VAS12,13. It also includes the presence of specific variables at cutaneous (ulceration, panniculitis, erythematous rash, periungual capillary changes, alopecia, mechanic’s hands, cutaneous vasculitis, sclerodactyly [localised thickening and hardening of the skin of the fingers and toes], Raynaud’s phenomenon and others), skeletal (arthritis and arthralgia), gastrointestinal (dysphagia or dysmotility), pulmonary (diffusing capacity for carbon monoxide [DLCO], forced vital capacity [FVC], interstitial lung disease patterns on high-resolution computed tomography [HRCT], chronic home oxygen requirement) and cardiac (pericarditis, myocarditis and arrhythmias) levels. Finally, it includes the physician and patient/parent or guardian global disease assessment at the time of the visit (0−10-cm VAS), and Manual Muscle Testing in eight muscle groups (MMT-8)12,13.
- 4
Functional capacity
The scores are collected of the Health Assessment Questionnaire for functional status in adults, and of the childhood HAQ (CHAQ relating to the week prior to the visit12,13.
- 5
Damage
Damage means persistent changes in anatomy, physiology, pathology, or function of any cause since the onset of myositis and present for at least six months. The Myositis Damage Index (MDI) is included, used by the clinician to assess the severity of damage by 10-cm VAS in 11 organs/systems (muscular, skeletal, cutaneous, gastrointestinal, pulmonary, cardiovascular, peripheral vascular, endocrine, ocular, infection, and malignancy). The global assessment of the severity of damage (0−10-cm VAS) of the MDI is also recorded12,13. The presence and date of diagnosis of specific variables of damage at cutaneous (calcinosis, lipodystrophy), pulmonary (pulmonary arterial hypertension), cardiovascular (arterial hypertension, ischaemic heart disease), peripheral vascular (venous or arterial thrombosis), endocrine (diabetes mellitus), serious infection (including location), malignant neoplasms (including type), mortality (including cause) levels are recorded. Finally, the physician and patient/parent or guardian global assessment of damage at the time of the visit (0−10 cm VAS) is included)12,13.
- 6
Laboratory
Muscle enzyme levels (CPK, GOT, GPT and LDH), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and ferritin are collected.
- 7
Antibodies
Information is collected on the presence of specific myositis-associated antibodies (anti-synthetase, anti-MDA5, anti-Mi-2, anti-SRP, anti-TIF1-γ, anti-NXP2, anti-HMG-CoA reductase, anti-SAE, anti-Ku, anti-Ro, anti-RNP, anti-PM/SCL).
- 8
Other comorbidities
Information is added on comorbidities that are not included in the damage variables (dyslipidaemia, smoking and association with other autoimmune diseases.
- 9
Hospitalisation
This includes information on hospitalisations and admissions to intensive care units (date and cause).
- 10
Treatment
The main treatments used for this disease and their indication are included: glucocorticoids, antimalarials, synthetic immunosuppressants, biological and synthetic targeted immunosuppressants, intravenous immunoglobulins and plasmapheresis (start date, end date and reason for discontinuation). Other less common treatments such as lung transplantation or direct haemoperfusion with polymyxin B are also included.
- 11
Quality of life
Information from the 12-Item Short-Form Health Survey (SF-12) quality of life measure is collected12,13.
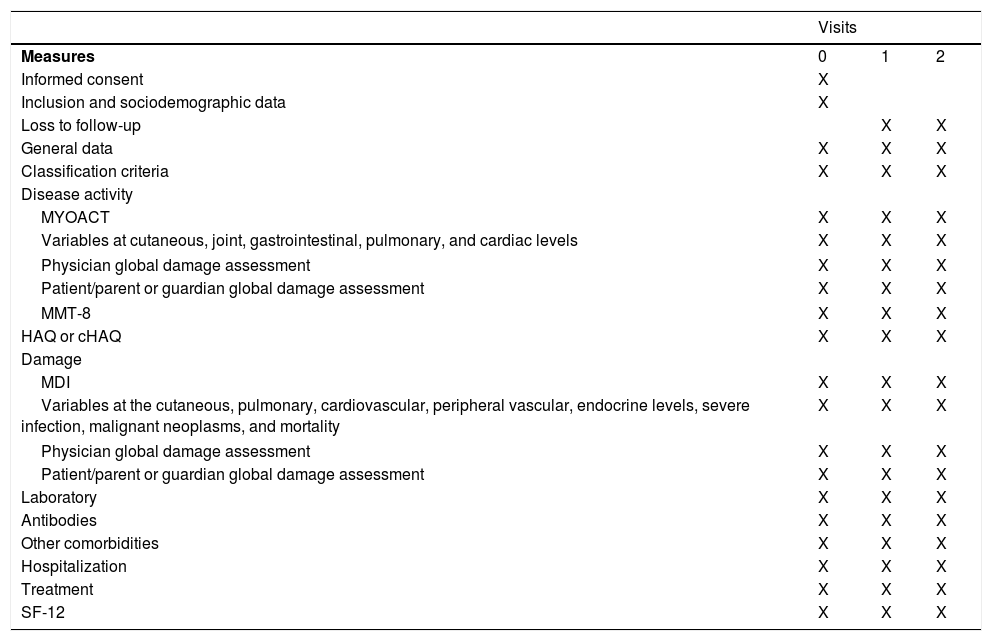
Table 2 shows the measures taken at each visit of the study.
Measures taken at each visit of the study.
| Visits | |||
|---|---|---|---|
| Measures | 0 | 1 | 2 |
| Informed consent | X | ||
| Inclusion and sociodemographic data | X | ||
| Loss to follow-up | X | X | |
| General data | X | X | X |
| Classification criteria | X | X | X |
| Disease activity | |||
| MYOACT | X | X | X |
| Variables at cutaneous, joint, gastrointestinal, pulmonary, and cardiac levels | X | X | X |
| Physician global damage assessment | X | X | X |
| Patient/parent or guardian global damage assessment | X | X | X |
| MMT-8 | X | X | X |
| HAQ or cHAQ | X | X | X |
| Damage | |||
| MDI | X | X | X |
| Variables at the cutaneous, pulmonary, cardiovascular, peripheral vascular, endocrine levels, severe infection, malignant neoplasms, and mortality | X | X | X |
| Physician global damage assessment | X | X | X |
| Patient/parent or guardian global damage assessment | X | X | X |
| Laboratory | X | X | X |
| Antibodies | X | X | X |
| Other comorbidities | X | X | X |
| Hospitalization | X | X | X |
| Treatment | X | X | X |
| SF-12 | X | X | X |
cHAQ: child HAQ; HAQ: Health Assessment Questionnaire; MDI: Myositis Damage Index; MYOACT: Myositis Disease Activity Assessment; MMT-8: Manual Muscle Testing of eight muscle groups; SF-12: 12-Item Short-Form Health Survey.
The sample size was not estimated, taking the objectives of the registry into account. All patients from each participating centre who meet the inclusion criteria are anticipated to be included in the study. Based on a previous study of interested centres, the total number of patients is estimated to be around 400. One hundred and eighty-six patients have been included in the first year of recruitment.
Quality control of the databaseAnnual online monitoring will take place. Each year, the consistency of all new data recorded in the data collection platform will be reviewed, paying special attention to the variables that gather information relating to the study objectives. Only patients who meet >80% of the visit variables will be considered eligible for analysis.
The procedures have been standardised in addition to the monitoring process. This standardisation has been included in a Myo-Spain investigator's manual available to all investigators participating in the study.
Visit reminderPeriodic (every two to three months) reminder and project status emails are sent to all participating centres during the inclusion period (V0). In addition, a reminder email is sent systematically from the online DCF to each centre’s investigators, notifying them closely in advance (in the month prior to the theoretical date) of the follow-up visits (V1 and V2), to ensure that the visits take place and patient information is gathered.
DiscussionThis paper describes the methodology of the Myo-Spain registry. The project represents the creation of a Spanish multicentre cohort of incident and prevalent patients with any type of IIM, with a prospective two-year follow-up.
Several registries have been established at the international level to investigate different aspects of IIM. The multicentre EuroMyositis registry was created in 1999 and currently involves more than 20 countries, primarily in Europe. The registry seeks to obtain uniform longitudinal data on juvenile and adult myositis to gain a better understanding of the course and prognosis of the disease. There is a mandatory data collection area containing basic information present at some time over the duration of the myositis, and another area, which is not mandatory to complete, corresponding to individual visits to monitor the course of the disease (activity and damage, muscle weakness, treatment, antibodies, and biopsy results). The registry has more than 3000 participants and publications have studied associations between clinical subtypes, extramuscular involvement, environmental exposure, antibodies, and drugs14,15. In addition, this cohort together with the UK and Ireland cohort, the Juvenile Dermatomyositis Research Group (JDRG), validated the new EULAR/ACR classification criteria11,16.
The American Myositis Patient Association launched the MYOVISION national registry in 2010. From December 2010 to July 2012, 1806 patients with IIM (PM, DM, IBM) were included in this registry and agreed to complete a paper or online survey with 83 questions on demographics, work or school, leisure activities, environmental exposure, disease exposure and health-related quality of life. Several cross-sectional studies on environmental exposure and quality of life have been published17,18. From the US multicentre Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, which included patients with childhood rheumatic diseases between 2010 and 2014, cross-sectional studies of the 688-patient juvenile DM cohort have been published on clinical characteristics and the association between exposure to ultraviolet radiation and disease severity19,20. Because of the limitations in the number of variables, in the information on treatments, and in the data collection process that was not systematic at each visit, the new CARRA registry, which was limited to juvenile idiopathic arthritis, was created in 201521.
The American and European NEtwork of Antisynthetase Syndrome (AENEAS) collaborative cohort is part of an international, multicentre, retrospective study, started in 2014, which includes patients with antisynthetase syndrome with at least six months’ follow-up. The publications in this cohort have focused on the form of presentation of the disease, progression, outcomes, and its specific behaviour, according to the presence of the different anti-synthetase antibodies or specific manifestations such as arthritis22–24.
We should highlight three initiatives at the national level. The first was internal medicine’s registry of patients with anti-synthetase syndrome of the Systemic Autoimmune Diseases Study Group (GEAS), which provided information on manifestations and long-term outcomes in patients with anti-jo1 antibodies25. The second was the registry of patients with inflammatory myopathy of the Madrid Rheumatology Society (REMICAM), with the participation of 12 rheumatology units of the Community of Madrid and retrospective collection of clinical and mortality data for patients under follow-up at some point between 1980 and December 2014. This registry has provided information on mortality, long-term pulmonary outcomes, overlap syndromes with myositis and juvenile forms26–29. Finally, the national registry of patients with MDA5 syndrome (MEDRA5), a multicentre and multidisciplinary registry that aims to understand the natural history of MDA5 syndrome, and has collected clinical data on these patients retrospectively until December 2019.
As a strength of the project, and unlike most of the abovementioned registries, Myo-Spain will carry out a prospective protocolised follow-up with annual visits of all patients. This was initially planned for two years, but is expected to be longer, given that IIM are infrequent and heterogeneous diseases and greater follow-up is required to determine their progression and management (the main objective of the project). All registry variables have been standardised, the data will be strictly monitored and patients who do not reach 80% of the required variables will be excluded from analysis. Although data from follow-up visits can also be collected prospectively in the EuroMyositis registry, this is not mandatory, and the incidence of new manifestations over time may be underestimated. In addition, in the early years of the registry, not all current subtypes of IIM, such as IMNM, had been established and some myositis-specific antibodies had not been discovered, and therefore a percentage of patients may be incorrectly classified. Another strength of Myo-Spain is that from the outset it was designed with two cohorts, one with incident patients (<12 months from diagnosis) and another with prevalent patients (>12 months from diagnosis), in which all patients must be alive and under active follow-up to be included. As this is a rare disease, we decided on an extended recruitment period, increasing the likelihood of including incident cases. At the same time, we aimed to determine disease activity, damage, disability, muscle strength and quality of life using validated indices and questionnaires to assess disease status and look for associations in a more objective way. Furthermore, by including all the subtypes of IIM, we will be able to make comparisons and identify differences in our population. We will also be able to study the diagnostic usefulness of the new classification criteria in this cohort of the future classification criteria for anti-synthetase syndrome and to determine the differences between patients with complete and incomplete forms at onset of the disease.
However, our registry also has a few limitations. First, it was not designed to be representative of the geographical distribution of IIM at the national level. This cohort does not include patients from all autonomous communities; to be specific, there are no data from centres in Aragón, Asturias, Cantabria, Castilla La Mancha, La Rioja, and Navarra. Another possible limitation is patient selection bias, including patients who may have an easier follow-up or who have fewer comorbidities. To avoid this bias, the need to recruit all patients who meet the inclusion criteria per centre was considered, which also becomes a necessity due to the prevalence and incidence data of IIM. Recruitment of all patients meeting the inclusion criteria has been reinforced through regular communication with participating investigators and at meetings at the start of the study and follow-up. Although this is a project of the Spanish Society of Rheumatology and the principal investigators at each centre are rheumatologists, to minimise possible recruitment bias, it was opened for the participation of collaborating investigators from other specialties such as internal medicine and neurology.
ConclusionsThe Myo-Spain study represents a cohort of patients with any type of IIM recruited in rheumatology units throughout Spain. This is the first prospective study of these characteristics that the SER has promoted in a rare disease. The cohort will include a group of incident patients and a group of prevalent patients, which will allow detailed assessment of the clinical characteristics of the disease at different times. It is expected that the comprehensive information collected at the visits will provide a valuable source of data for future analysis and research.
FundingMyo-Spain is funded by Kern Pharma (Spain), Nordic Pharma (Spain), Sandoz (Spain) and Bristol-Myers Squibb (Spain). None of these companies were involved in the study design, data collection or analysis, or in drafting this article.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Fernando Sánchez-Alonso and Nuria Montero, staff members of the Research Unit of the Spanish Society of Rheumatology who are collaborating in the development of the project.
Please cite this article as: Cobo-Ibáñez T, Sánchez-Piedra C, Nuño-Nuño L, Castellví I, Carrión-Barberà I, Romero-Bueno F, et al. Myo-Spain: Registro de pacientes con miopatía inflamatoria idiopática de España. Metodología. Reumatol Clín. 2022;18:253–259.