To describe the objectives, design and methods of the Spanish Society of Rheumatology systemic lupus erythematosus (SLE) registry (RELESSER).
MethodsMulticenter, hospital-based registry, with retrospective collection of data from a large representative sample of adult patients with SLE (1997 ACR criteria) attending Spanish rheumatology services. The registry includes demographic data, frequent and infrequent (<1%) clinical manifestations, information about activity, damage, severity, comorbidity, treatments and mortality, collecting 359 variables per patient, with highly standardized definitions. We performed a preliminary descriptive analysis of the data.
ResultsForty-five centers were involved and 4024 SLE patients (91% with ≥4 ACR criteria) have been included; 90% are women and 93% Caucasians, with a median age at diagnosis of 33 years, median disease duration: 120 months, median follow-up duration: 104 months; 3222 (81%) of the patients are in active follow-up and 591 (14%) were lost to follow-up. The median values of the SELENA-SLEDAI score, SLICC/ACR damage index and Katz severity index have been 2, 1 and 2, respectively. A total of 211 patients (6%) died.
ConclusionsRELESSER represents the largest European SLE registry built to date, providing comprehensive and reliable information on SLE manifestations, disease status, comorbid conditions and treatments in daily clinical practice. RELESSER is constituted as a tool of great potential for multicenter clinical research in SLE.
Describir en detalle los objetivos y aspectos metodológicos del registro de lupus eritematoso sistémico (LES) de la Sociedad Española de Reumatología (RELESSER).
MétodosRegistro multicéntrico, de base hospitalaria, con recogida retrospectiva de datos de una amplia muestra representativa de adultos con LES (criterios ACR 1997) procedentes de servicios de reumatología españoles. Incluye datos demográficos, manifestaciones clínicas frecuentes e infrecuentes (<1%), actividad, daño, gravedad, comorbilidad, tratamientos y mortalidad, totalizando 359 variables por paciente, con definiciones altamente estandarizadas. Se ha realizado un análisis descriptivo preliminar de los datos.
ResultadosHan participado 45 centros e incluido 4024 pacientes con LES o LES incompleto (91% con ≥ 4 criterios ACR). El 90% son mujeres y el 93% caucásicos, con una mediana de edad al diagnóstico de 33 años; mediana de duración de la enfermedad: 120 meses; seguimiento medio: 104 meses. Se encuentran en seguimiento activo 3.222 pacientes (81%) y 591 (14%) han sido perdidos para seguimiento. Las medianas del índice de actividad SELENA-SLEDAI, índice de daño de SLICC/ACR y de gravedad de Katz han sido 2, 1 y 2, respectivamente. Un total de 211 pacientes (6%) han fallecido.
ConclusionesRELESSER representa el registro de LES europeo con mayor número de pacientes construido hasta la fecha, disponiendo de abundante información actualizada y fiable sobre manifestaciones del LES, situación de enfermedad, comorbilidad y tratamientos en condiciones de práctica clínica real. RELESSER se constituye como herramienta de gran potencialidad para la investigación clínica multicéntrica en el LES.
Systemic lupus erythematosus (SLE) is one of the most common systemic rheumatic diseases, with an increasing incidence.1 In Spain, the prevalence is estimated at 9 per 10000 population.2,3 Although the prognosis has improved in recent years, the quality of life of patients with SLE is clearly lower than in the general population4 and the risk of death is 2–3 times higher.5,6 In addition, SLE has important direct and indirect health costs.7
Clinical manifestations of SLE, its course and prognosis are highly heterogeneous and, given its low prevalence, multicenter observational studies are needed with a high degree of standardization and sufficient number of patients, to advance the understanding of this complex disease. Registries allow large sample sizes, recruitment of unselected patients in ‘real’ clinical practice conditions, and longer follow-up than clinical trials. This allows a better evaluation of outcomes such as damage, comorbidity and mortality, as well as comparative analysis of subgroups of patients.8–11 It is no wonder, therefore, that multicenter registries of patients with SLE and their cohorts derivatives have become a fundamental tool in clinical research on this disease.12–14 Moreover, as demonstrated in the Lupus in Minorities: Nature versus Nurture (LUMINA),15,16 Grupo Latinoamericano Lupus Study (GLADEL)17 and other cohorts,18–20 there is a large ethnic variability in the clinical manifestations of SLE, in severity and even the response to immunosuppressive treatments,21 which is not yet well defined. Not surprisingly, a study of the severity of SLE in different European populations is one of the priority research areas proposed by a group of experts from EULAR.22 In Spain there are few studies of patients with SLE to provide consistent data, due to their local nature or by the limited sample size or short follow-up.23–26 Clinical characteristics and peculiarities of SLE in the whole country are not precisely known, nor is its comorbidity, severity, mortality and its causes, nor comorbidity. In particular, there are no data on the current management of SLE, the percentage of patients refractory to treatment or the degree of implementation of certain therapies, such as immunosuppressants and antimalarials, which have shown long-term benefits.27,28 Another patient population for which there are no data in our area is that of incomplete SLE, understood as those patients who do not meet the four minimum classification criteria of the American College of Rheumatology (ACR) but who receive a diagnosis of SLE, with no other disease to explain the clinical picture.29
In order to try to answer some of these questions and to promote multicenter clinical research on SLE, the Spanish Society of Rheumatology (SER) has launched, supporting the initiative of the working group for Systemic Autoimmune Diseases (EAS-SER) and with the methodological support and supervision of the Research Unit of the SER (UI-SER), a registry of systemic lupus erythematosus of the SER (RELESSER).
RELESSER is a multicenter registry of patients with SLE which consists of 2 phases, a transversal one (RELESSER-T), for which the inclusion of patients has been already completed, and a longitudinal prospective study conducted on a selected sample of patients included in an initial cohort (RELESSER-PROS).
This paper describes the objectives, design and methodology of RELESSER-T, some of the basic descriptive results and the strengths and weaknesses of the registry.
Patients and MethodsDesign of the Systemic Lupus Erythematosus Registry of the Spanish Society of Rheumatology, Transverse PhaseMulticenter retrospective hospital-based registry of adult patients with SLE, with electronic collection of data from medical records review.
ObjectivesThe main objectives of RELESSER-T are:
- (1)
To describe the sociodemographic and clinical and laboratory characteristics of patients with SLE and incomplete SLE (LESI) (< 4 ACR criteria) seen in rheumatology departments in our country, especially those with unusual clinical manifestations (<1%), as well as the status of the disease, severity, treatment, complications and morbidity.
- (2)
To establish a comprehensive and consistent record of well-characterized patients, from which to select subgroups on which to develop longitudinal studies that allow us to answer different research questions, constituting RELESSER-PROS.
As secondary objectives, the registry is to contribute to improve the level of instruction on systematic and standardized assessment of the disease, its implementation in daily clinical practice and promote cooperative multicenter clinical SLE research among Spanish rheumatologists.
Patient Recruitment and Data AcquisitionUnselected patients ≥16 years, diagnosed with SLE (regardless of vital status), according to the revised criteria (1997).30,31 ACR, considered “defined SLE” (Lesd), or patients with only 3 criteria but SLE diagnosed according to the clinical judgment of an expert rheumatologist, called Lesi in this record. All patients were from the hospital rheumatology services of the public health system (except one private center) with active members of EAS-SER. We planned to include at least 80% of patients in follow-up (with more than one visit to rheumatology) at some point in each center. We excluded those patients who did not have least 50% of the data, a criteria defined as “minimum essential data” (comprising a total of 151 variables) (Table 1). The recruitment period was set at 10 months. In order to minimize missing data (“missing values”) and optimize the representation, a pre-screening visit was established 3 months before the start of enrollment period, which allowed us to complete the census of patients in each hospital, recovering SLE patients not identified as such and supplementing the local databases, making missing values recoverable.
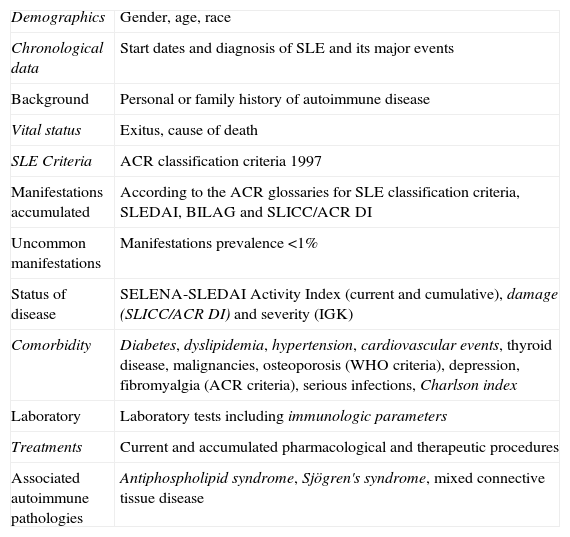
Variables Included in RELESSER.
| Demographics | Gender, age, race |
| Chronological data | Start dates and diagnosis of SLE and its major events |
| Background | Personal or family history of autoimmune disease |
| Vital status | Exitus, cause of death |
| SLE Criteria | ACR classification criteria 1997 |
| Manifestations accumulated | According to the ACR glossaries for SLE classification criteria, SLEDAI, BILAG and SLICC/ACR DI |
| Uncommon manifestations | Manifestations prevalence <1% |
| Status of disease | SELENA-SLEDAI Activity Index (current and cumulative), damage (SLICC/ACR DI) and severity (IGK) |
| Comorbidity | Diabetes, dyslipidemia, hypertension, cardiovascular events, thyroid disease, malignancies, osteoporosis (WHO criteria), depression, fibromyalgia (ACR criteria), serious infections, Charlson index |
| Laboratory | Laboratory tests including immunologic parameters |
| Treatments | Current and accumulated pharmacological and therapeutic procedures |
| Associated autoimmune pathologies | Antiphospholipid syndrome, Sjögren's syndrome, mixed connective tissue disease |
ACR: American College of Rheumatology; BILAG: British Isles Lupus Assessment Group; IGK: Katz severity index, SLE: systemic lupus erythematosus; WHO: World Health Organization; SELENA-SLEDAI: Safety of Estrogens in Systemic Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index, SLICC/ACR DI: Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index.
In italics, variables considered “essential” for the record.
Data collection in each center was made by rheumatologists with experience in the diagnosis and treatment of patients with SLE. To ensure consistency in the collection, we created an operations manual for the transversal registry phase (procedures, instructions for the proper use of indexes, glossaries with precise definitions of each variable, the instruction and explanatory remarks for the manual and electronic database, etc.), and carried out a preliminary exercise, specifically designed for recording, via the Internet, the way in which we evaluated the agreement in the use of assessment indexes of disease among different researchers and the gold standard, using clinical cases. The gold standard for correct responses was established by the two principal investigators (PI) (IRF and JMPR), both certified in the use of these indices by the Lupus Foundation of America, following a review of the same by the scientific committee of the registry. Unsolvable discrepancies using the definition of the items were resolved by consensus between the PI and the contributing researcher. We also established a forum, via the Internet, which allows direct interaction with the PI. The source document has been the clinical history of patients.
We created an online electronic application, specific for the registry, without any data collection on notebooks or paper, in order to minimize transcription errors and speed up the analysis process. We have avoided as far as possible free text responses and composite scores, the latter being directly performed by the application. The application contains filters, non-surpassable ranges, help menus and dialogs to maximize the reliability of the data. Prior to the start of data collection under the supervision of the UI-SER, we conducted a pilot study that involved five centers outside those of the PIs, reviewing 4 patients per center, with the purpose of testing the platform and identifying possible errors, inconsistencies or clarifying uncertainties that may arise during use.
The first patient was included on 27-10-2011 and the last on 13-08-2012, with effective inclusion having a duration of 10 months. Once entered, the data were reviewed and edited and we proceeded to block the electronic data collection notebooks to prevent further editing.
MonitoringThis was performed via the Internet, conducted by a qualified, accredited instructor, experienced in the field of rheumatology. For this, and using the statistical program Stata®, inconsistencies were monitored, described as missing (e.g. Age, Gender), logical (e.g. active monitoring state but with a cause of death), reviewed between different modules of the electronic notebook, inclusion criteria (e.g. <16) and strict adherence to the criteria for the SLE damage index, activity, severity and other definitions in the protocol. Everything has been reviewed by the PI and agreed upon with the monitor after several inconsistencies detection reviews by the panel. The inconsistencies of all centers were sent as discrepancies to the investigators concerned and were followed up until complete resolution.
VariablesWe included 359 variables per patient, grouped as follows: (1) demographics, (2) chronological, (3) general clinical data, including vital status, (4) cumulative manifestations of SLE, defined by the glossaries of the ACR criteria for classification of SLE30,31 and the activity indexes Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)32 and British Isles Lupus Assessment Group (BILAG) index33,34 and the damage Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index (SLICC/ACR DI),35 (5) the general SLE activity index, Systemic Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)-SLEDAI,36 damage index (SLICC/ACR DI)35 and severity index (Katz index) (IGK),37 (6) rare events (<1%), (7) comorbidity, including infections requiring hospitalization or cause of death (causal agent, location and treatment at the time of infection) and the Charlson comorbidity index, as amended by Deyo,38 (8) laboratory testing, (9) imaging or histological evidence when they were needed; (10) treatments, cause of discontinuation as per case (clinical problem solved, ineffectiveness, or other adverse reaction) (Table 1).
Antiphospholipid syndrome was defined according to the Sydney criteria,39 mixed connective tissue disease by the criteria proposed by Alarcon-Segovia40 and Sjögren's syndrome in the presence of compatible sicca syndrome and positive Schirmer test, or biopsy/salivary gland scintigraphy. SLE was considered refractory if one or more of the following criteria was satisfied: inefficiency of cyclophosphamide, ineffectiveness of at least 2 of the following immunosuppressive treatments: methotrexate, leflunomide, azathioprine, mycophenolate, Rituximab or splenectomy, at any time during the course of the disease and whatever manifestation had led to their use. Unusual events were recorded (prevalence <1% in the literature), not included in the aforementioned definition, by variables per device list plus a free text section.
The information was collected as present if it had been at any time of evolution (cumulative incidence). In the case of treatments, we also collected them if present at last available assessment. We collected the presence of the variables that constitute the SELENA-SLEDAI activity index at any time during the course of the disease and in the 10 days prior to the latest assessment of the patient.
We created a codebook with clear and unambiguous operational definitions, to guide the coding process and to facilitate the location of variables and interpretation of the data during analysis.
Statistical AnalysisBased on a prevalence of approximately 1% (the minimum expected between the manifestations of SLE that we attempted to estimate), an accuracy of 2% and a confidence interval of 95% we calculated a minimum sample size of 815 patients. However, we decided to include the largest possible number of patients in each hospital in order to get a record that was as comprehensive as possible. We estimated, based on a previous survey (on possible losses and other variables), a sample of 2500 target patients, sufficient to achieve the main objective of the registry and enable subgroup analyzes.
We conducted a basic descriptive analysis using tables of absolute and relative frequencies of qualitative variables, position measurements means, median and measures of dispersion (SD: standard deviation, IR: interquartile range).
Ethical AspectsThe project complies with the principles of the Declaration of Helsinki41 and the Oviedo Convention.42 Confidentiality has been respected in accordance with Royal Decree 1720/2007 and Law 15/199943 for Data Protection. The project has obtained authorization from the managements of the participating centers and approval by a Research Ethics Committee (CEIC) and the reference CEIC each center, as per required. All researchers involved have signed a document showing the commitment of the researcher.
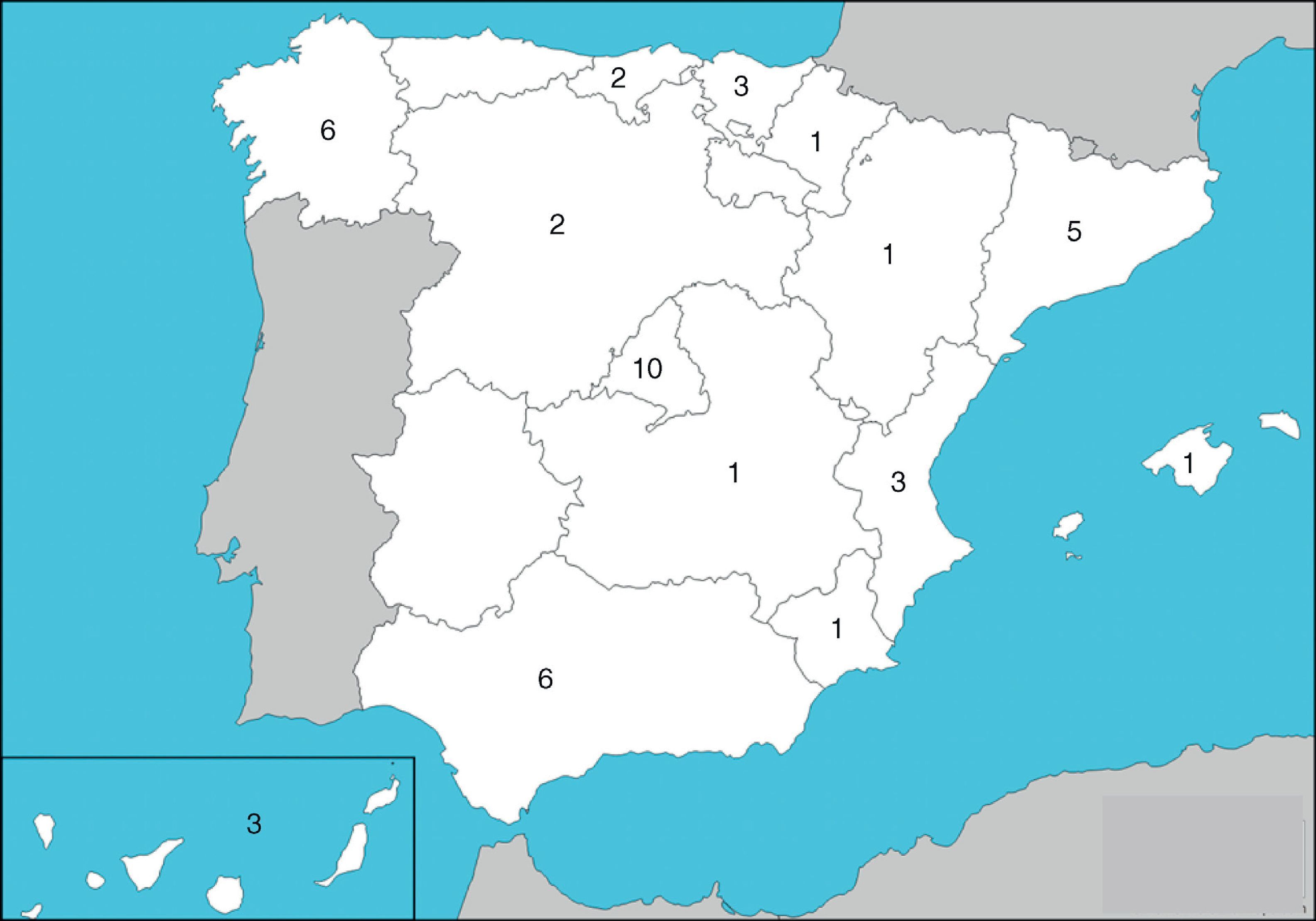
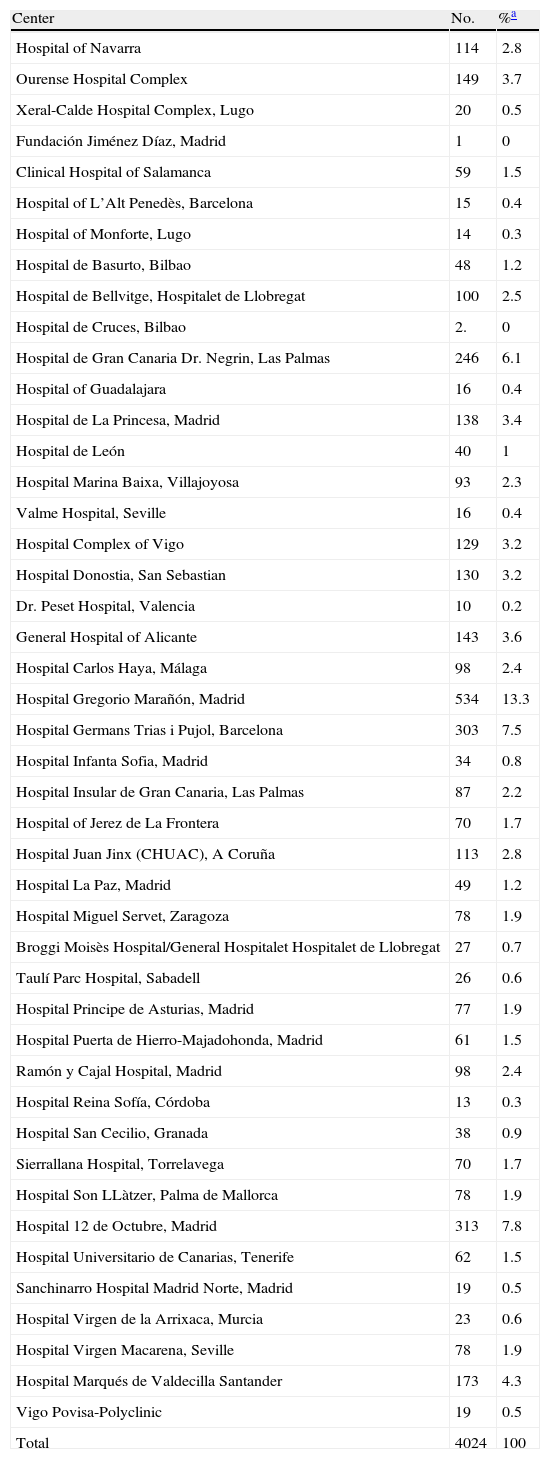
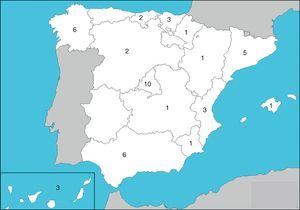
ResultsHospitals and Distribution of Patients45 hospitals were involved, with representation from almost all of the regions (Fig. 1) care levels and dimensions. The average number of patients included per center was 89±99, range: 1–534. Most centers (n=41.91%)≤ contributed 5% of the hospital patients and only one exceeded 10% of the total (Table 2).
Participating Centers and Patients Included per Center.
| Center | No. | %a |
| Hospital of Navarra | 114 | 2.8 |
| Ourense Hospital Complex | 149 | 3.7 |
| Xeral-Calde Hospital Complex, Lugo | 20 | 0.5 |
| Fundación Jiménez Díaz, Madrid | 1 | 0 |
| Clinical Hospital of Salamanca | 59 | 1.5 |
| Hospital of L’Alt Penedès, Barcelona | 15 | 0.4 |
| Hospital of Monforte, Lugo | 14 | 0.3 |
| Hospital de Basurto, Bilbao | 48 | 1.2 |
| Hospital de Bellvitge, Hospitalet de Llobregat | 100 | 2.5 |
| Hospital de Cruces, Bilbao | 2. | 0 |
| Hospital de Gran Canaria Dr. Negrin, Las Palmas | 246 | 6.1 |
| Hospital of Guadalajara | 16 | 0.4 |
| Hospital de La Princesa, Madrid | 138 | 3.4 |
| Hospital de León | 40 | 1 |
| Hospital Marina Baixa, Villajoyosa | 93 | 2.3 |
| Valme Hospital, Seville | 16 | 0.4 |
| Hospital Complex of Vigo | 129 | 3.2 |
| Hospital Donostia, San Sebastian | 130 | 3.2 |
| Dr. Peset Hospital, Valencia | 10 | 0.2 |
| General Hospital of Alicante | 143 | 3.6 |
| Hospital Carlos Haya, Málaga | 98 | 2.4 |
| Hospital Gregorio Marañón, Madrid | 534 | 13.3 |
| Hospital Germans Trias i Pujol, Barcelona | 303 | 7.5 |
| Hospital Infanta Sofia, Madrid | 34 | 0.8 |
| Hospital Insular de Gran Canaria, Las Palmas | 87 | 2.2 |
| Hospital of Jerez de La Frontera | 70 | 1.7 |
| Hospital Juan Jinx (CHUAC), A Coruña | 113 | 2.8 |
| Hospital La Paz, Madrid | 49 | 1.2 |
| Hospital Miguel Servet, Zaragoza | 78 | 1.9 |
| Broggi Moisès Hospital/General Hospitalet Hospitalet de Llobregat | 27 | 0.7 |
| Taulí Parc Hospital, Sabadell | 26 | 0.6 |
| Hospital Principe de Asturias, Madrid | 77 | 1.9 |
| Hospital Puerta de Hierro-Majadohonda, Madrid | 61 | 1.5 |
| Ramón y Cajal Hospital, Madrid | 98 | 2.4 |
| Hospital Reina Sofía, Córdoba | 13 | 0.3 |
| Hospital San Cecilio, Granada | 38 | 0.9 |
| Sierrallana Hospital, Torrelavega | 70 | 1.7 |
| Hospital Son LLàtzer, Palma de Mallorca | 78 | 1.9 |
| Hospital 12 de Octubre, Madrid | 313 | 7.8 |
| Hospital Universitario de Canarias, Tenerife | 62 | 1.5 |
| Sanchinarro Hospital Madrid Norte, Madrid | 19 | 0.5 |
| Hospital Virgen de la Arrixaca, Murcia | 23 | 0.6 |
| Hospital Virgen Macarena, Seville | 78 | 1.9 |
| Hospital Marqués de Valdecilla Santander | 173 | 4.3 |
| Vigo Povisa-Polyclinic | 19 | 0.5 |
| Total | 4024 | 100 |
4024 patients were included: 3679 (91%) with SLE and 345 (9%) with Lesi. 3.222 patients (81%) were actively followed and 591 (14%) were lost to follow-up at the time of inclusion in the registry. The initial average percentage of “do not follow” patients (relative to overall follow-up time in rheumatology), defined as the time from the onset of the first criterion of SLE until the first assessment for rheumatology service, was 27.4% (SD: 29.2%). 50% of patients have been followed for 14.2% of the time their disease with an IR (3.1%–47.5%). We established the coincidence in date of birth and gender as a mechanism to identify possible duplicates.
Only in 7 of the 359 variables collected≥were more than 20% of the values missing (family history of autoimmune disease, menopause, use of contraceptives, anti β 2 glycoprotein I IgG and IgM, lupus anticoagulant and high creatinine). In 92% of the variables, the percentage of losses has been less than 5%.
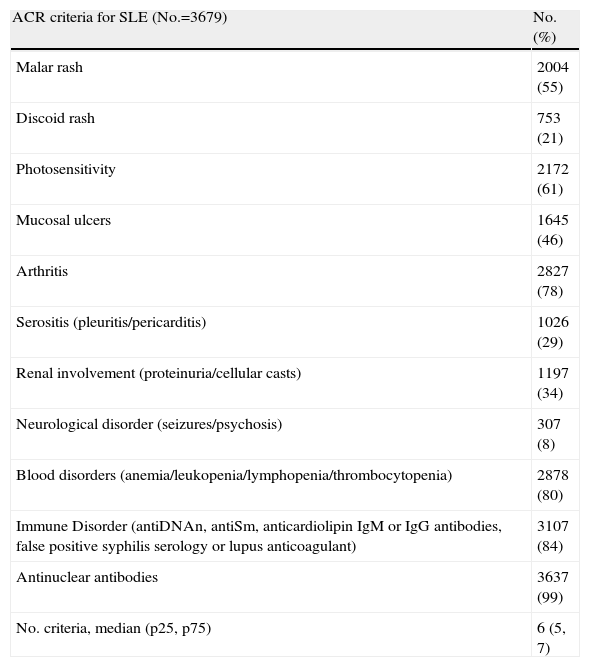
Basic Descriptive ResultsDemographic data are shown in Table 3. 90% of patients are women, 93% Caucasian (n=3905), median (IR) age at diagnosis of 33 [25–45] years, median (IR) duration of SLE, since diagnosis, 120 [60.201] months. Table 4 shows the percentage of patients who presented each of the ACR classification criteria. Comorbidities are shown in Table 5.
Demographic and Chronological Variables (Patients With ≥4 ACR Criteria).
| Characteristic (No.=3679) | No. (%) |
| Gender | |
| Male | 357 (10) |
| Female | 3315 (90) |
| Age>50 years at the time of diagnosis | 569 (16) |
| Characteristic (No.=3679) | Median (p25, p75) |
| Age at onset of first symptom (years) | 31 (22:42) |
| Age at diagnosis of SLE (years) | 33 (24.44) |
| Age at last assessment (years) | 45 (36.57) |
| Duration of SLE (months) | 124 (64.205) |
| Delay in diagnosis (months) | 9 (3.33) |
| Time followed in rheumatology (months) | 104 (9.172) |
ACR: American College of Rheumatology; SLE: systemic lupus erythematosus.
Cumulative SLE Criteria (Patients With ≥4 ACR Criteria).
| ACR criteria for SLE (No.=3679) | No. (%) |
| Malar rash | 2004 (55) |
| Discoid rash | 753 (21) |
| Photosensitivity | 2172 (61) |
| Mucosal ulcers | 1645 (46) |
| Arthritis | 2827 (78) |
| Serositis (pleuritis/pericarditis) | 1026 (29) |
| Renal involvement (proteinuria/cellular casts) | 1197 (34) |
| Neurological disorder (seizures/psychosis) | 307 (8) |
| Blood disorders (anemia/leukopenia/lymphopenia/thrombocytopenia) | 2878 (80) |
| Immune Disorder (antiDNAn, antiSm, anticardiolipin IgM or IgG antibodies, false positive syphilis serology or lupus anticoagulant) | 3107 (84) |
| Antinuclear antibodies | 3637 (99) |
| No. criteria, median (p25, p75) | 6 (5, 7) |
ACR: American College of Rheumatology; SLE: systemic lupus erythematosus.
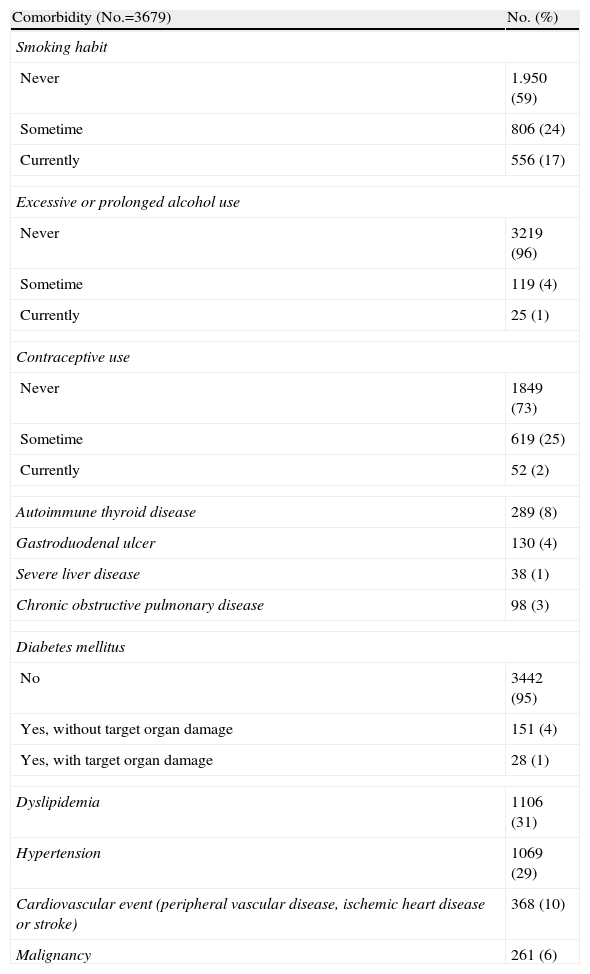
Comorbidities in Patients With ≥4 ACR Criteria for SLE.
| Comorbidity (No.=3679) | No. (%) |
| Smoking habit | |
| Never | 1.950 (59) |
| Sometime | 806 (24) |
| Currently | 556 (17) |
| Excessive or prolonged alcohol use | |
| Never | 3219 (96) |
| Sometime | 119 (4) |
| Currently | 25 (1) |
| Contraceptive use | |
| Never | 1849 (73) |
| Sometime | 619 (25) |
| Currently | 52 (2) |
| Autoimmune thyroid disease | 289 (8) |
| Gastroduodenal ulcer | 130 (4) |
| Severe liver disease | 38 (1) |
| Chronic obstructive pulmonary disease | 98 (3) |
| Diabetes mellitus | |
| No | 3442 (95) |
| Yes, without target organ damage | 151 (4) |
| Yes, with target organ damage | 28 (1) |
| Dyslipidemia | 1106 (31) |
| Hypertension | 1069 (29) |
| Cardiovascular event (peripheral vascular disease, ischemic heart disease or stroke) | 368 (10) |
| Malignancy | 261 (6) |
ACR: American College of Rheumatology; SLE: systemic lupus erythematosus.
The median (IR) activity level in patients with SLE (No.=3679), according to SELENA-SLEDAI is 2 [0.4], median (IR) of SLICC/ACR DI: 1 [0.2] and median (IR) of the IGK 2 [1,3]. For patients being currently followed (No.=3222), medium (RI) values of the above indices are very similar: 2 [0.4] 0 [0.1] and 2 [1 3] respectively. The median (IR) of the Charlson comorbidity index was 2 (1–3) on a scale of 0–37. A total of 211 patients included (6%) died, 55 (26%) due to SLE, 53 (25%) infections, 53 (25%) cardiovascular events and 32 (15%) due to cancer.
DiscussionIn the clinical study of SLE, registries provide data from a larger number of patients in non-experimental situations, so they complement controlled studies.10,12 Despite their limited epidemiological interest,44 hospital records can be of great use to analyze the magnitude and distribution of disease manifestations in actual practice conditions, increasing their knowledge and the burden of disease, contributing to improve health planning.11
Following EULAR45 and the Agency for Healthcare Research and Quality (AHRQ) recommendations,11 the RELESSER project justification is set to be a registry, its feasibility and raised research questions are intended to respond to the transverse phase thereof, with well-defined objectives. Sample size has been estimated analyzing potential confounders. The population included is ethnically homogeneous and has an acceptable registry representativeness (external validity). Despite using a sample of hospital origin, we expected that a large majority of patients diagnosed with SLE in our country have been evaluated at some time in a hospital, minimizing selection bias by type of hospital. Most patients are in active monitoring, by which we mean that the data obtained acceptably reflect current clinical reality of SLE in Spain. The sample size achieved, along with the final inclusion of at least 70% of the patients identified in the administrative or clinical databases vast majority of participating centers, increases external validity.
A minimum core of essential variables to be collected was established and clear definitions of each variable established through the use of standardized definitions, of broad international level, to be linked mostly to activity and damage indices commonly used in research. Application was disposed to maximize consistency and minimize errors of interpretation and participants were trained, thus ensuring the high level of data quality of RELESSER-T.46
RELESSER-T has finally included a total of 4024 cases of SLE, becoming the largest European cohort gathered so far. This number far exceeds the sample size required to capture enough cases of rare events, facilitating and enabling accurate characterization for subgroup analysis, particularly relevant in a disease as heterogeneous as SLE.
RELESSER-T contains updated and comprehensive information on SLE, unpublished data such as the prevalence of refractory disease activity and severity or ‘burden’ of disease, Charlson comorbidity index or the large number of unusual events not previously recognized in large cohorts of patients with SLE. It will also allow the study of rare events and severe infectious complications and cardiovascular comorbidity in our environment. RELESSER-T is thus a powerful tool for multicenter clinical research, either from data already collected or to seek additional information in order to respond to new research questions.12,47
The registry can be affected by certain selection bias related to the method of choice for centers with specialists interested in clinical research in SLE. It is possible that patients of participating centers are more seriously ill or have been better studied. There may also be differences in referral to rheumatology depending on various factors such as the level of care or the presence of certain organ-specific manifestations that encourage referral to other specialties. But the sample size, the large number of participating centers and their wide geographic distribution and the characteristics of the participating centers minimize selection bias.
Another limitation may lie in the incomplete follow-up, from the beginning of the disease, in the rheumatology clinic, partly caused by the delay in diagnosis. However, we interpret that the period of absence of monitoring by a rheumatology service at the beginning of the disease has been relatively brief, and we believe that the loss of information is probably minor.
However, the main and most obvious limitation of RELESSER-T is its retrospective nature, tempered by a low percentage of missing values, despite the large number of variables. The limitations inherent in studies based on such registries are well known, including a higher frequency of measurement error and restriction of information on potential confounders.
The present study shows some basic, very preliminary descriptive data of RELESSER-T. Specific analyses are underway on activity, refractory disease, damage, morbidity, mortality, infection and renal SLE. RELESSER-T will also allow a comparative analysis of SLE and Lesi, and examine racial differences (Caucasians and Hispanics) or peculiarities of male patients or those with a disease onset after 50 years of age, among other potential subgroups, each with enough patients as to obtain reliable conclusions. In the future, from RELESSER-T we will create prospective cohorts of SLE patients, in order to try to confirm associations of different variables with damage, mortality, and other outcome measures established in multivariate analyzes of transversal registries, exploring its predictive nature.
Conclusions- 1.
RELESSER-T represents the largest European registry of patients with SLE to date, having rich, detailed and highly reliable data, still in the process of analysis, on common and uncommon manifestations of SLE, level of activity, damage, comorbidity and treatments commonly used in actual clinical practice conditions.
- 2.
RELESSER is a powerful tool in the face of multicenter clinical research in SLE and its peculiarities in southern Europe.
The authors declare that no experiments have been performed on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace on the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors state that no patient data appear in this article.
FinancingGSK, Roche, UCB, Novartis.
Conflict of InterestThe authors declare no conflict of interest.
To Loreto Carmona, for her wise counsel and methodological contributions; Sabina Perez, for her contribution in statistical aspects; Juan Manuel Barrio for his computer work, essential for the development of the platform, and to all SER staff who has participated in a way or another in the project.
Inmaculada de la Torre Ortega (Hospital Gregorio Marañón, Madrid). Esther Rodríguez-Almaraz (Hospital Doce de Octubre, Madrid). Esteban Salas Heredia, Gregorio Santos Soler, José Carlos Rosas Gómez de Salazar, Carlos Santos Ramírez y José M. Senabre Gallego (Hospital de Marina Baixa, Villajoyosa, Alicante). Paloma Vela Casasempere, Mariano Andrés Collado y José Antonio Bernal (Hospital General de Alicante). Mónica Ibáñez Barcelo, Imaculada Ros Vilamajó, Antonio Juan Mas y Claudia Murillo (Hospital Son Llàtzer, Palma de Mallorca). Vicente Torrente Segarra (Hospital Moisés Broggi/Hospital General Hospitalet, Hospitalet de Llobregat). Ivan Castellví Barranco (Hospital Comarcal de L’Alt Penedés, Barcelona). Javier Narváez García (Hospital de Bellvitge, Hospitalet de LLobregat). Alejandro Olivé Marqués y Emma García Melchor (Hospital Germans Trias i Pujol, Badalona). Joan Calvet Fontova, María García Manrique, Carlos Galisteo Lencastre y Mireia Moreno Martínez-Losa (Hospital Parc Taulí, Sabadell) Raúl Menor Almagro (Hospital Jerez de La Frontera). Ricardo Blanco Alonso, Víctor Martínez Taboada, Miguel A. González-Gay Mantecón, Inés Pérez Martín y M. del Carmen Bejerano (Hospital Marqués de Valdecilla, Santander). Ignacio Villa Blanco, Begoña Moreira, Elena Aurrecoechea y Teresa Ruiz Jimeno (Hospital Sierrallana, Torrelavega, Cantabria). Ángeles Aguirre Zamorano (Hospital Reina Sofía, Córdoba). Mercedes Freire González (Hospital Juan Canalejo, A Coruña). César Magro y Enrique Raya Álvarez (Hospital San Cecilio, Granada). Celia Erausquin Arruabarrena y M. Ángeles Acosta Mérida (Hospital Dr. Negrín, Las Palmas de Gran Canaria). Esther Uriarte Isacelaya, Cesar A. Egües Dubuc y Jorge Cancio Fanlo (Hospital de Donosti, San Sebastián). Elvira Díez Álvarez, Carlos Vitovi y Alejandra López Robles (Hospital de León). Tomás Vázquez Rodríguez (Hospital Lucus Augusti, Lugo). Antonio Fernández-Nebro, Mª Victoria Irigoyen Oyarzábal, Mª Ángeles Belmonte López y Carmen Mª Romero Barco (Hospital Carlos Haya, Málaga). Juan Antonio Martínez López y Olga Sánchez Pernaute (Fundación Jiménez Díaz, Madrid). Eva Tornero Muriel y Txaro Gª de Vicuña Pinedo (Hospital de la Princesa, Madrid). Marta Valero Expósito, Paloma García de la Peña, Silvia Rodríguez Rubio y Jorge J. González Martín (Hospital Univ. Madrid Norte Sanchinarro, Madrid). Ana Pérez Gómez, Cristina Bohorquez, Atusa Morasat Hajkhan, Ana I. Turrión Nieves y Ana Sánchez Atrio (Hospital Príncipe de Asturias, Madrid). José Luis Andreu Sánchez, Lucía Silva Fernández y Mónica Fdez. de Castro (Hospital Puerta de Hierro, Madrid). Antonio Zea Mendoza, Ana J. Lois Iglesias, Aline Lucice Boteanu y Mª Luz Gamir Gamir (Hospital Ramón y Cajal, Madrid). Patricia Richi Alberti y Santiago Muñoz Fernández (Hospital Infanta Sofía, Madrid). Gema Bonilla Hernán (Hospital La Paz, Madrid). María Rosario Oliva y Carlos Marras Fdez. Cid (Hospital Virgen de la Arrixaca, Murcia). Concepción Fito Manteca, Claudia Stoye N del P y Loreto Horcada Rubio (Complejo Hospitalario de Navarra). Mª Teresa Otón Sánchez, Iñigo Hernández Rodríguez y Coral Mouriño Rodríguez (Hospital Meixoeiro, Vigo). Carlos A. Montilla Morales y Ruth López González (Hospital Clínico Univ. de Salamanca). Blanca Hernández Cruz, Federico Navarro Sarabia y Francisco J. Toyos Sáenz de Miera (Hospital Virgen Macarena, Sevilla). José Luis Marenco de la Fuente, Julia Uceda Montañés, Raquel Hernández Sánchez y Rosalía Martínez Pérez (Hospital de Valme, Sevilla). Beatriz Rodríguez Lozano y Marian Gantes Mora (Hospital Univ. de Canarias, Tenerife). Eduardo Úcar Angulo, Mª Esther Ruiz Lucea y Olaia Fernández Berrizbeitia (Hospital de Basurto, Bilbao). Luis López Domínguez (Hospital de Cruces, Barakaldo, Bilbao). Juan J. Alegre Sancho, Isabel de La Morena Barrio y Elia Valls (Hospital Dr. Peset, Valencia). Ángela Pecondón Español y Javier Manero Ruiz (Hospital Miguel Servet, Zaragoza). Jesús Ibáñez Ruán (Clínica POVISA, Vigo). Manuel Rodríguez Gómez y Rafael B. Melero González (Hospital de Orense). Víctor E. Quevedo Vila (Hospital de Monforte de Lemos, Lugo). Sergio Machín, José A. Hernández Beriain y Javier Nóvoa (Hospital Insular de Gran Canaria). Lucia Silva Fernández (Hospital de Guadalajara).
Please cite this article as: Rúa-Figueroa I, López-Longo FJ, Calvo-Alén J, Galindo-Izquierdo M, Loza E, García de Yebenes MJ, et al. Registro nacional de pacientes con lupus eritematoso sistémico de la Sociedad Española de Reumatología: objetivos y metodología. Reumatol Clin. 2014;10:17–24.
The EAS-SER members, collaborators and other researchers participating in the Registry are listed at the end of the document.